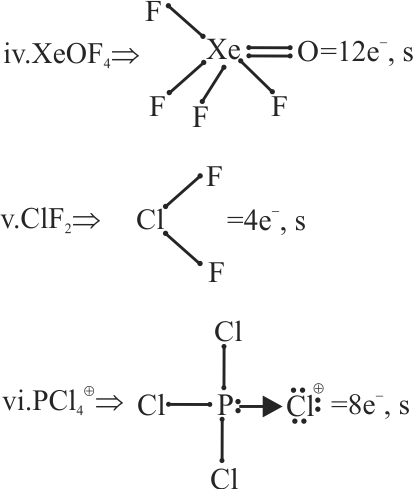313795
Read the Statement - A and Statement - B carefully to mark the correct options given below:
Statement A :
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule.
Statement B :
Octet rule explains the formation of compounds of inert gases such as
\(\mathrm{XeF}_2, \mathrm{XeO}_4\) etc.
313796
How many of the following compounds violate octet rule?
\[\begin{array}{*{20}{l}}
{{\rm{(i)Br}}{{\rm{F}}_{\rm{5}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(ii)S}}{{\rm{F}}_{\rm{6}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(iii)I}}{{\rm{F}}_{\rm{7}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(iv)XeO}}{{\rm{F}}_{\rm{4}}}}\\
{{\rm{(v)Cl}}{{\rm{F}}_{\rm{2}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(vi)PC}}{{\rm{l}}_{\rm{4}}}^ \oplus }
\end{array}\]
313795
Read the Statement - A and Statement - B carefully to mark the correct options given below:
Statement A :
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule.
Statement B :
Octet rule explains the formation of compounds of inert gases such as
\(\mathrm{XeF}_2, \mathrm{XeO}_4\) etc.
313796
How many of the following compounds violate octet rule?
\[\begin{array}{*{20}{l}}
{{\rm{(i)Br}}{{\rm{F}}_{\rm{5}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(ii)S}}{{\rm{F}}_{\rm{6}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(iii)I}}{{\rm{F}}_{\rm{7}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(iv)XeO}}{{\rm{F}}_{\rm{4}}}}\\
{{\rm{(v)Cl}}{{\rm{F}}_{\rm{2}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(vi)PC}}{{\rm{l}}_{\rm{4}}}^ \oplus }
\end{array}\]
313795
Read the Statement - A and Statement - B carefully to mark the correct options given below:
Statement A :
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule.
Statement B :
Octet rule explains the formation of compounds of inert gases such as
\(\mathrm{XeF}_2, \mathrm{XeO}_4\) etc.
313796
How many of the following compounds violate octet rule?
\[\begin{array}{*{20}{l}}
{{\rm{(i)Br}}{{\rm{F}}_{\rm{5}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(ii)S}}{{\rm{F}}_{\rm{6}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(iii)I}}{{\rm{F}}_{\rm{7}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(iv)XeO}}{{\rm{F}}_{\rm{4}}}}\\
{{\rm{(v)Cl}}{{\rm{F}}_{\rm{2}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(vi)PC}}{{\rm{l}}_{\rm{4}}}^ \oplus }
\end{array}\]
313795
Read the Statement - A and Statement - B carefully to mark the correct options given below:
Statement A :
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule.
Statement B :
Octet rule explains the formation of compounds of inert gases such as
\(\mathrm{XeF}_2, \mathrm{XeO}_4\) etc.
313796
How many of the following compounds violate octet rule?
\[\begin{array}{*{20}{l}}
{{\rm{(i)Br}}{{\rm{F}}_{\rm{5}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(ii)S}}{{\rm{F}}_{\rm{6}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(iii)I}}{{\rm{F}}_{\rm{7}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(iv)XeO}}{{\rm{F}}_{\rm{4}}}}\\
{{\rm{(v)Cl}}{{\rm{F}}_{\rm{2}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(vi)PC}}{{\rm{l}}_{\rm{4}}}^ \oplus }
\end{array}\]
313795
Read the Statement - A and Statement - B carefully to mark the correct options given below:
Statement A :
The Lewis dot structures provide a picture of bonding in molecules and ions in terms of the shared pairs of electrons and the octet rule.
Statement B :
Octet rule explains the formation of compounds of inert gases such as
\(\mathrm{XeF}_2, \mathrm{XeO}_4\) etc.
313796
How many of the following compounds violate octet rule?
\[\begin{array}{*{20}{l}}
{{\rm{(i)Br}}{{\rm{F}}_{\rm{5}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(ii)S}}{{\rm{F}}_{\rm{6}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(iii)I}}{{\rm{F}}_{\rm{7}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(iv)XeO}}{{\rm{F}}_{\rm{4}}}}\\
{{\rm{(v)Cl}}{{\rm{F}}_{\rm{2}}}{\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\mkern 1mu} {\rm{(vi)PC}}{{\rm{l}}_{\rm{4}}}^ \oplus }
\end{array}\]