CHXI04:CHEMICAL BONDING AND MOLECULAR STRUCTURE
313632
Here (a) and (b) are hydrogen bond and covalent bonds, their lengths are
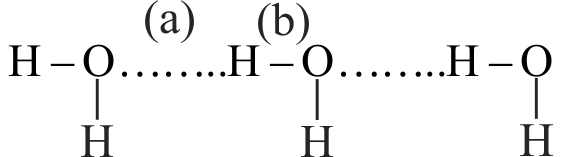
1 \({\rm{0}}{\rm{.97}}{{\rm{A}}^{\rm{o}}}{\rm{,0}}{\rm{.97}}{{\rm{A}}^{\rm{o}}}\)
2 \({\rm{1}}{\rm{.73}}{{\rm{A}}^{{\rm{o,}}}}{\rm{0}}{\rm{.97}}{{\rm{A}}^{\rm{o}}}\)
3 \({\rm{1}}{\rm{.73}}{{\rm{A}}^{\rm{o}}}{\rm{,1}}{\rm{.73}}{{\rm{A}}^{\rm{o}}}\)
4 \({\rm{0}}{\rm{.97}}{{\rm{A}}^{\rm{o}}}{\rm{,1}}{\rm{.73}}{{\rm{A}}^{\rm{o}}}\)
Explanation:
Covalent bond length is less than hydrogen bond.