313635
The correct statement/s about hydrogen bonding is/ are
(I) Hydrogen bonding exists when H is covalently bonded to the highly electronegative atom.
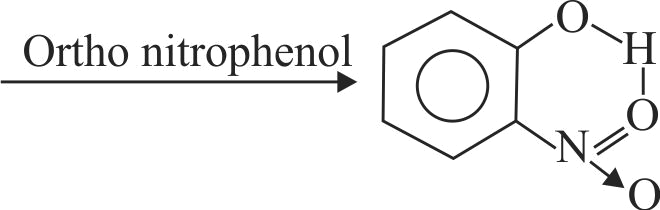
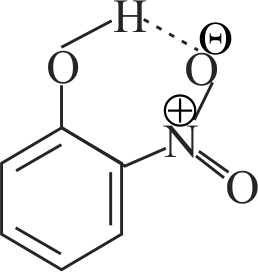
(II) Intermolecular H - bonding is present in o-nitro phenol.
(III) Intramolecular H -bonding is present in HF
(IV) The magnitude of H -bonding depends on the physical state of the compound.
(V) H-bonding has powerful effect on the structure and properties of compounds.
Choose the correct answer from the options given below :
313635
The correct statement/s about hydrogen bonding is/ are
(I) Hydrogen bonding exists when H is covalently bonded to the highly electronegative atom.
(II) Intermolecular H - bonding is present in o-nitro phenol.
(III) Intramolecular H -bonding is present in HF
(IV) The magnitude of H -bonding depends on the physical state of the compound.
(V) H-bonding has powerful effect on the structure and properties of compounds.
Choose the correct answer from the options given below :
313635
The correct statement/s about hydrogen bonding is/ are
(I) Hydrogen bonding exists when H is covalently bonded to the highly electronegative atom.
(II) Intermolecular H - bonding is present in o-nitro phenol.
(III) Intramolecular H -bonding is present in HF
(IV) The magnitude of H -bonding depends on the physical state of the compound.
(V) H-bonding has powerful effect on the structure and properties of compounds.
Choose the correct answer from the options given below :
313635
The correct statement/s about hydrogen bonding is/ are
(I) Hydrogen bonding exists when H is covalently bonded to the highly electronegative atom.
(II) Intermolecular H - bonding is present in o-nitro phenol.
(III) Intramolecular H -bonding is present in HF
(IV) The magnitude of H -bonding depends on the physical state of the compound.
(V) H-bonding has powerful effect on the structure and properties of compounds.
Choose the correct answer from the options given below :
313635
The correct statement/s about hydrogen bonding is/ are
(I) Hydrogen bonding exists when H is covalently bonded to the highly electronegative atom.
(II) Intermolecular H - bonding is present in o-nitro phenol.
(III) Intramolecular H -bonding is present in HF
(IV) The magnitude of H -bonding depends on the physical state of the compound.
(V) H-bonding has powerful effect on the structure and properties of compounds.
Choose the correct answer from the options given below :