CHXI04:CHEMICAL BONDING AND MOLECULAR STRUCTURE
313563
Assertion :
bond in ethyne is shorter than bonds in ethene.
Reason :
( Carbon atom in ethene is hybridised while it is in ethyne.
1 Both Assertion and Reason are correct and Reason is the correct explanation of the Assertion.
2 Both Assertion and Reason are correct but Reason is not the correct explanation of the Assertion.
3 Assertion is correct but Reason is incorrect.
4 Both Assertion and Reason are incorrect
Explanation:
The carbon atoms in ethene is hybridised due to
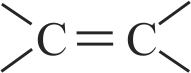
t while it is sp in ethyne due to
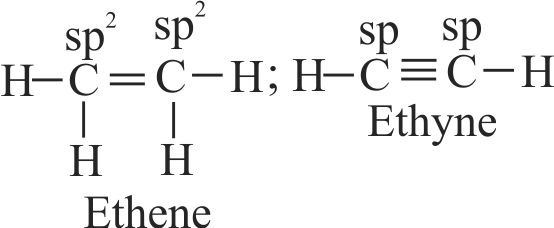
Hence, Assertion is correct but Reason is incorrect.