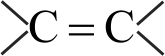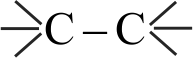313478
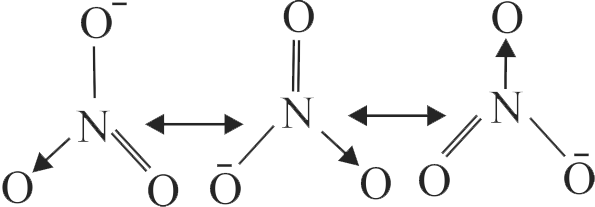
Increasing order of carbon-carbon bond length for the following is
\({{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}\,\,\,\,\,{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{2}}}\,\,\,\,\,{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}\,\,\,\,\,{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{6}}}\)
\(\,\left( {\rm{A}} \right)\,\,\,\,\,\,\,\,\,\left( {\rm{B}} \right)\,\,\,\,\,\,\,\,\left( {\rm{C}} \right)\,\,\,\,\,\,\,\,\,\left( {\rm{D}} \right)\)
313478
Increasing order of carbon-carbon bond length for the following is
\({{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}\,\,\,\,\,{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{2}}}\,\,\,\,\,{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}\,\,\,\,\,{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{6}}}\)
\(\,\left( {\rm{A}} \right)\,\,\,\,\,\,\,\,\,\left( {\rm{B}} \right)\,\,\,\,\,\,\,\,\left( {\rm{C}} \right)\,\,\,\,\,\,\,\,\,\left( {\rm{D}} \right)\)
313478
Increasing order of carbon-carbon bond length for the following is
\({{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}\,\,\,\,\,{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{2}}}\,\,\,\,\,{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}\,\,\,\,\,{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{6}}}\)
\(\,\left( {\rm{A}} \right)\,\,\,\,\,\,\,\,\,\left( {\rm{B}} \right)\,\,\,\,\,\,\,\,\left( {\rm{C}} \right)\,\,\,\,\,\,\,\,\,\left( {\rm{D}} \right)\)
313478
Increasing order of carbon-carbon bond length for the following is
\({{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}\,\,\,\,\,{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{2}}}\,\,\,\,\,{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{\rm{6}}}\,\,\,\,\,{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{6}}}\)
\(\,\left( {\rm{A}} \right)\,\,\,\,\,\,\,\,\,\left( {\rm{B}} \right)\,\,\,\,\,\,\,\,\left( {\rm{C}} \right)\,\,\,\,\,\,\,\,\,\left( {\rm{D}} \right)\)