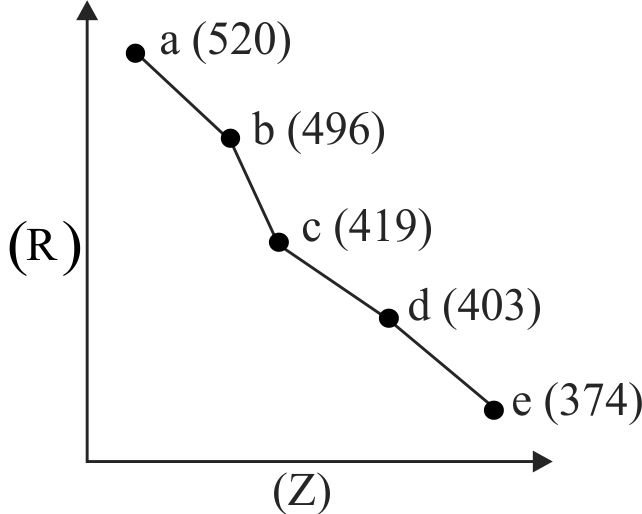
313390
Consider the following elements.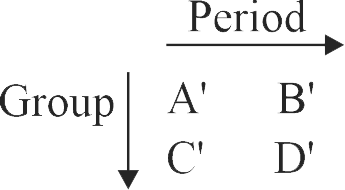
Which of the following is/are true about \({\mathrm{\mathrm{A}^{\prime}, \mathrm{B}^{\prime}, \mathrm{C}^{\prime}}}\) and \({\mathrm{\mathrm{D}^{\prime}}}\) ?
(I) Order of atomic radii : \({\mathrm{\mathrm{B}^{\prime} < \mathrm{A}^{\prime} < \mathrm{D}^{\prime} < \mathrm{C}^{\prime}}}\)
(II) Order of metallic character :\({\mathrm{B^{\prime} < A^{\prime} < D^{\prime} < C^{\prime}}}\)
(III) Size of the elements: \({\mathrm{\mathrm{D}^{\prime} < \mathrm{C}^{\prime} < \mathrm{B}^{\prime} < \mathrm{A}^{\prime}}}\)
(IV) Order of ionic radii : \({{\rm{B}}^{\prime {\rm{ + }}}} < {{\rm{A}}^{^{\prime {\rm{ + }}}}} < {{\rm{D}}^{\prime {\rm{ + }}}} < {{\rm{C}}^{\prime {\rm{ + }}}}\)
Choose the correct answer from the options given below:
313390
Consider the following elements.
Which of the following is/are true about \({\mathrm{\mathrm{A}^{\prime}, \mathrm{B}^{\prime}, \mathrm{C}^{\prime}}}\) and \({\mathrm{\mathrm{D}^{\prime}}}\) ?
(I) Order of atomic radii : \({\mathrm{\mathrm{B}^{\prime} < \mathrm{A}^{\prime} < \mathrm{D}^{\prime} < \mathrm{C}^{\prime}}}\)
(II) Order of metallic character :\({\mathrm{B^{\prime} < A^{\prime} < D^{\prime} < C^{\prime}}}\)
(III) Size of the elements: \({\mathrm{\mathrm{D}^{\prime} < \mathrm{C}^{\prime} < \mathrm{B}^{\prime} < \mathrm{A}^{\prime}}}\)
(IV) Order of ionic radii : \({{\rm{B}}^{\prime {\rm{ + }}}} < {{\rm{A}}^{^{\prime {\rm{ + }}}}} < {{\rm{D}}^{\prime {\rm{ + }}}} < {{\rm{C}}^{\prime {\rm{ + }}}}\)
Choose the correct answer from the options given below:
313390
Consider the following elements.
Which of the following is/are true about \({\mathrm{\mathrm{A}^{\prime}, \mathrm{B}^{\prime}, \mathrm{C}^{\prime}}}\) and \({\mathrm{\mathrm{D}^{\prime}}}\) ?
(I) Order of atomic radii : \({\mathrm{\mathrm{B}^{\prime} < \mathrm{A}^{\prime} < \mathrm{D}^{\prime} < \mathrm{C}^{\prime}}}\)
(II) Order of metallic character :\({\mathrm{B^{\prime} < A^{\prime} < D^{\prime} < C^{\prime}}}\)
(III) Size of the elements: \({\mathrm{\mathrm{D}^{\prime} < \mathrm{C}^{\prime} < \mathrm{B}^{\prime} < \mathrm{A}^{\prime}}}\)
(IV) Order of ionic radii : \({{\rm{B}}^{\prime {\rm{ + }}}} < {{\rm{A}}^{^{\prime {\rm{ + }}}}} < {{\rm{D}}^{\prime {\rm{ + }}}} < {{\rm{C}}^{\prime {\rm{ + }}}}\)
Choose the correct answer from the options given below:
313390
Consider the following elements.
Which of the following is/are true about \({\mathrm{\mathrm{A}^{\prime}, \mathrm{B}^{\prime}, \mathrm{C}^{\prime}}}\) and \({\mathrm{\mathrm{D}^{\prime}}}\) ?
(I) Order of atomic radii : \({\mathrm{\mathrm{B}^{\prime} < \mathrm{A}^{\prime} < \mathrm{D}^{\prime} < \mathrm{C}^{\prime}}}\)
(II) Order of metallic character :\({\mathrm{B^{\prime} < A^{\prime} < D^{\prime} < C^{\prime}}}\)
(III) Size of the elements: \({\mathrm{\mathrm{D}^{\prime} < \mathrm{C}^{\prime} < \mathrm{B}^{\prime} < \mathrm{A}^{\prime}}}\)
(IV) Order of ionic radii : \({{\rm{B}}^{\prime {\rm{ + }}}} < {{\rm{A}}^{^{\prime {\rm{ + }}}}} < {{\rm{D}}^{\prime {\rm{ + }}}} < {{\rm{C}}^{\prime {\rm{ + }}}}\)
Choose the correct answer from the options given below: