313390
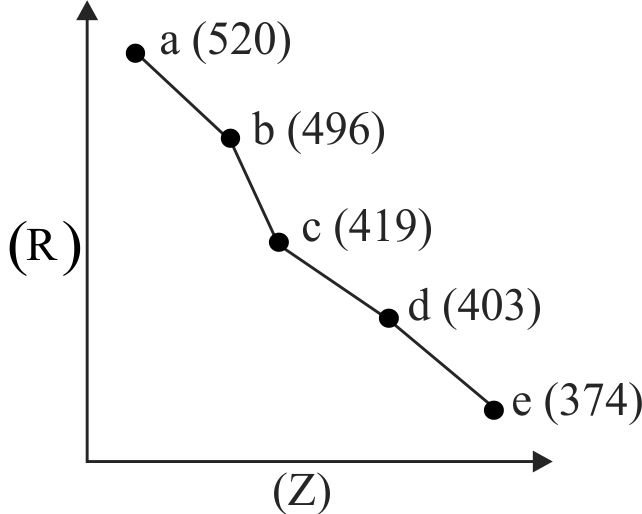
Consider the following elements.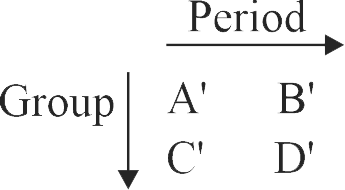
Which of the following is/are true about
(I) Order of atomic radii :
(II) Order of metallic character :
(III) Size of the elements:
(IV) Order of ionic radii :
Choose the correct answer from the options given below:
313390
Consider the following elements.
Which of the following is/are true about
(I) Order of atomic radii :
(II) Order of metallic character :
(III) Size of the elements:
(IV) Order of ionic radii :
Choose the correct answer from the options given below:
313390
Consider the following elements.
Which of the following is/are true about
(I) Order of atomic radii :
(II) Order of metallic character :
(III) Size of the elements:
(IV) Order of ionic radii :
Choose the correct answer from the options given below:
313390
Consider the following elements.
Which of the following is/are true about
(I) Order of atomic radii :
(II) Order of metallic character :
(III) Size of the elements:
(IV) Order of ionic radii :
Choose the correct answer from the options given below: