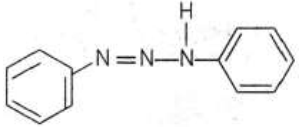278865
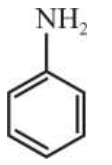
Three isomers $\mathbf{A}, \mathbf{B}$ and $\mathrm{C}$ (molecular formula
$\mathrm{C}_{8} \mathrm{H}_{11} \mathrm{~N}$ ) give the following results :
AandC \(\xrightarrow{\text { Diazotisation }} \mathbf{R}(\) Product of \(\mathbf{A})\)
\(\mathbf{P}+\mathbf{O} \xrightarrow[\substack{\text { (ii) Oxidation } \\\left(\mathrm{KMnO}_4+\mathrm{H}^{+}\right)}]{\text {(i) Hydrysis }} \mathbf{S}\) (Product of \(\mathbf{C})\)
\(R\) has lower boiling point than \(S\).
B \(\xrightarrow{\mathrm{C}_6 \mathrm{H}_5 \mathrm{SO}_2 \mathrm{Cl}}\) alkali - insoluble product
\(A, B\) and \(C\) respectively are
278865
Three isomers $\mathbf{A}, \mathbf{B}$ and $\mathrm{C}$ (molecular formula
$\mathrm{C}_{8} \mathrm{H}_{11} \mathrm{~N}$ ) give the following results :
AandC \(\xrightarrow{\text { Diazotisation }} \mathbf{R}(\) Product of \(\mathbf{A})\)
\(\mathbf{P}+\mathbf{O} \xrightarrow[\substack{\text { (ii) Oxidation } \\\left(\mathrm{KMnO}_4+\mathrm{H}^{+}\right)}]{\text {(i) Hydrysis }} \mathbf{S}\) (Product of \(\mathbf{C})\)
\(R\) has lower boiling point than \(S\).
B \(\xrightarrow{\mathrm{C}_6 \mathrm{H}_5 \mathrm{SO}_2 \mathrm{Cl}}\) alkali - insoluble product
\(A, B\) and \(C\) respectively are
278865
Three isomers $\mathbf{A}, \mathbf{B}$ and $\mathrm{C}$ (molecular formula
$\mathrm{C}_{8} \mathrm{H}_{11} \mathrm{~N}$ ) give the following results :
AandC \(\xrightarrow{\text { Diazotisation }} \mathbf{R}(\) Product of \(\mathbf{A})\)
\(\mathbf{P}+\mathbf{O} \xrightarrow[\substack{\text { (ii) Oxidation } \\\left(\mathrm{KMnO}_4+\mathrm{H}^{+}\right)}]{\text {(i) Hydrysis }} \mathbf{S}\) (Product of \(\mathbf{C})\)
\(R\) has lower boiling point than \(S\).
B \(\xrightarrow{\mathrm{C}_6 \mathrm{H}_5 \mathrm{SO}_2 \mathrm{Cl}}\) alkali - insoluble product
\(A, B\) and \(C\) respectively are
278865
Three isomers $\mathbf{A}, \mathbf{B}$ and $\mathrm{C}$ (molecular formula
$\mathrm{C}_{8} \mathrm{H}_{11} \mathrm{~N}$ ) give the following results :
AandC \(\xrightarrow{\text { Diazotisation }} \mathbf{R}(\) Product of \(\mathbf{A})\)
\(\mathbf{P}+\mathbf{O} \xrightarrow[\substack{\text { (ii) Oxidation } \\\left(\mathrm{KMnO}_4+\mathrm{H}^{+}\right)}]{\text {(i) Hydrysis }} \mathbf{S}\) (Product of \(\mathbf{C})\)
\(R\) has lower boiling point than \(S\).
B \(\xrightarrow{\mathrm{C}_6 \mathrm{H}_5 \mathrm{SO}_2 \mathrm{Cl}}\) alkali - insoluble product
\(A, B\) and \(C\) respectively are
278865
Three isomers $\mathbf{A}, \mathbf{B}$ and $\mathrm{C}$ (molecular formula
$\mathrm{C}_{8} \mathrm{H}_{11} \mathrm{~N}$ ) give the following results :
AandC \(\xrightarrow{\text { Diazotisation }} \mathbf{R}(\) Product of \(\mathbf{A})\)
\(\mathbf{P}+\mathbf{O} \xrightarrow[\substack{\text { (ii) Oxidation } \\\left(\mathrm{KMnO}_4+\mathrm{H}^{+}\right)}]{\text {(i) Hydrysis }} \mathbf{S}\) (Product of \(\mathbf{C})\)
\(R\) has lower boiling point than \(S\).
B \(\xrightarrow{\mathrm{C}_6 \mathrm{H}_5 \mathrm{SO}_2 \mathrm{Cl}}\) alkali - insoluble product
\(A, B\) and \(C\) respectively are