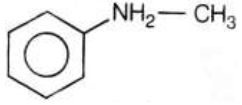
278846
Given below are two statements :
Statement I:
Primary aliphatic amines react with $\mathrm{HNO}_{2}$ to give unstable diazonium salts.
Statement II:
Primary aliphatic amines react with $\mathrm{HNO}_{2}$ to form diazonium salts which are stable even above $300 \mathrm{~K}$. On the light of the above statements, choose the most appropriate answer from the options given below:
278847 An organic compound ' $A$ ' contains nitrogen and chlorine. It dissolves readily in water to give a solution that turns litmus red. Titration of compound ' $A$ ' with standard base indicates that the molecular weight of ' $A$ ' is $131 \pm 2$. When a sample of ' $A$ ' is treated with aq. $\mathrm{NaOH}$, a liquid separates which contains $\mathrm{N}$ but not $\mathrm{Cl}$. Treatment' of the obtained liquid with nitrous acid followed by phenol gives orange precipitate. The compound ' $A$ ' is:
278846
Given below are two statements :
Statement I:
Primary aliphatic amines react with $\mathrm{HNO}_{2}$ to give unstable diazonium salts.
Statement II:
Primary aliphatic amines react with $\mathrm{HNO}_{2}$ to form diazonium salts which are stable even above $300 \mathrm{~K}$. On the light of the above statements, choose the most appropriate answer from the options given below:
278847 An organic compound ' $A$ ' contains nitrogen and chlorine. It dissolves readily in water to give a solution that turns litmus red. Titration of compound ' $A$ ' with standard base indicates that the molecular weight of ' $A$ ' is $131 \pm 2$. When a sample of ' $A$ ' is treated with aq. $\mathrm{NaOH}$, a liquid separates which contains $\mathrm{N}$ but not $\mathrm{Cl}$. Treatment' of the obtained liquid with nitrous acid followed by phenol gives orange precipitate. The compound ' $A$ ' is:
278846
Given below are two statements :
Statement I:
Primary aliphatic amines react with $\mathrm{HNO}_{2}$ to give unstable diazonium salts.
Statement II:
Primary aliphatic amines react with $\mathrm{HNO}_{2}$ to form diazonium salts which are stable even above $300 \mathrm{~K}$. On the light of the above statements, choose the most appropriate answer from the options given below:
278847 An organic compound ' $A$ ' contains nitrogen and chlorine. It dissolves readily in water to give a solution that turns litmus red. Titration of compound ' $A$ ' with standard base indicates that the molecular weight of ' $A$ ' is $131 \pm 2$. When a sample of ' $A$ ' is treated with aq. $\mathrm{NaOH}$, a liquid separates which contains $\mathrm{N}$ but not $\mathrm{Cl}$. Treatment' of the obtained liquid with nitrous acid followed by phenol gives orange precipitate. The compound ' $A$ ' is:
278846
Given below are two statements :
Statement I:
Primary aliphatic amines react with $\mathrm{HNO}_{2}$ to give unstable diazonium salts.
Statement II:
Primary aliphatic amines react with $\mathrm{HNO}_{2}$ to form diazonium salts which are stable even above $300 \mathrm{~K}$. On the light of the above statements, choose the most appropriate answer from the options given below:
278847 An organic compound ' $A$ ' contains nitrogen and chlorine. It dissolves readily in water to give a solution that turns litmus red. Titration of compound ' $A$ ' with standard base indicates that the molecular weight of ' $A$ ' is $131 \pm 2$. When a sample of ' $A$ ' is treated with aq. $\mathrm{NaOH}$, a liquid separates which contains $\mathrm{N}$ but not $\mathrm{Cl}$. Treatment' of the obtained liquid with nitrous acid followed by phenol gives orange precipitate. The compound ' $A$ ' is: