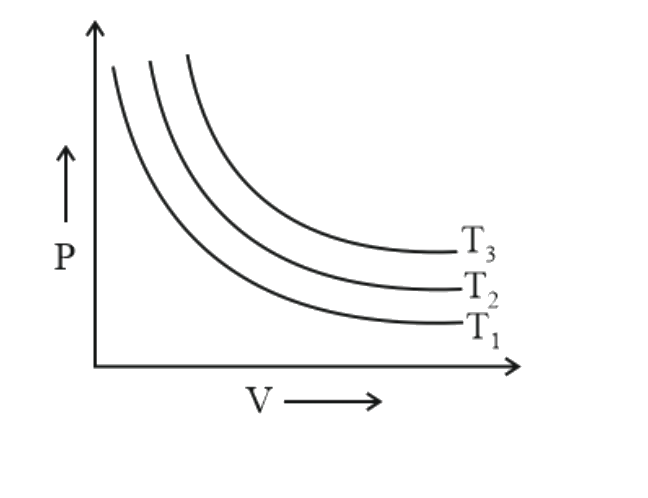
272425 An ideal gas initially at temperature, pressure and volume, $27^{\circ} \mathrm{C}, 1.00$ bar and $10 \mathrm{~L}$, respectively is heated at constant volume until pressure is 10.0 bar; it then undergoes a reversible isothermal expansion until pressure is 1.00 bar. What is the total work $W$, during this process?
272425 An ideal gas initially at temperature, pressure and volume, $27^{\circ} \mathrm{C}, 1.00$ bar and $10 \mathrm{~L}$, respectively is heated at constant volume until pressure is 10.0 bar; it then undergoes a reversible isothermal expansion until pressure is 1.00 bar. What is the total work $W$, during this process?
272425 An ideal gas initially at temperature, pressure and volume, $27^{\circ} \mathrm{C}, 1.00$ bar and $10 \mathrm{~L}$, respectively is heated at constant volume until pressure is 10.0 bar; it then undergoes a reversible isothermal expansion until pressure is 1.00 bar. What is the total work $W$, during this process?
272425 An ideal gas initially at temperature, pressure and volume, $27^{\circ} \mathrm{C}, 1.00$ bar and $10 \mathrm{~L}$, respectively is heated at constant volume until pressure is 10.0 bar; it then undergoes a reversible isothermal expansion until pressure is 1.00 bar. What is the total work $W$, during this process?
272425 An ideal gas initially at temperature, pressure and volume, $27^{\circ} \mathrm{C}, 1.00$ bar and $10 \mathrm{~L}$, respectively is heated at constant volume until pressure is 10.0 bar; it then undergoes a reversible isothermal expansion until pressure is 1.00 bar. What is the total work $W$, during this process?