371625
The two conducting cylinder-piston systems shown below are linked. Cylinder 1 is filled with a certain molar quantity of a monatomic ideal gas, and cylinder 2 is filled with an equal molar quantity of a diatomic ideal gas. The entire apparatus is situated inside an oven whose temperature is \({T_{a}=27^{\circ} {C}}\). The cylinder volumes have the same initial value \({V_{0}=100 {cc}}\). When the oven temperature is slowly raised to \({T_{b}=127^{\circ} {C}}\). The volume change \({\Delta V}\) (in \(cc\)) of cylinder 1 is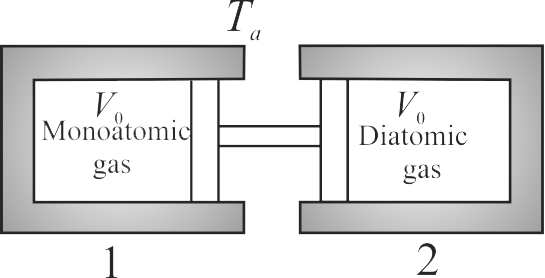
371626
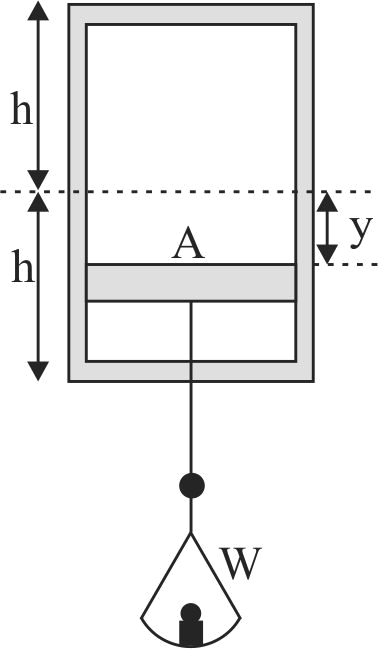
A horizontal frictionless piston, of negligible mass and heat capacity, divides a vertical insulated cylinder into two halves. Each half of the cylinder contains 1 mole of air at standard temperature and pressure \(p_{o}\). A load of weight \(W\) is now suspended from the piston, as shown in the figure. It pulls the piston down and comes to rest after a few oscillations. How large a volume does the compressed air in the lower part of the cylinder ultimately occupy if \(W\) is very large?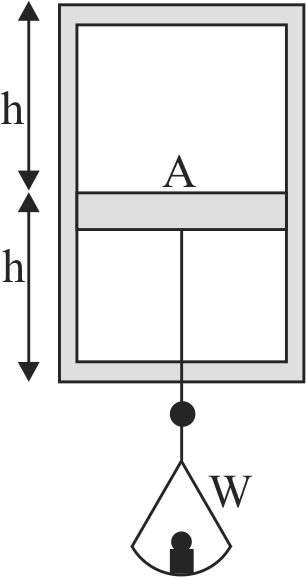
371627
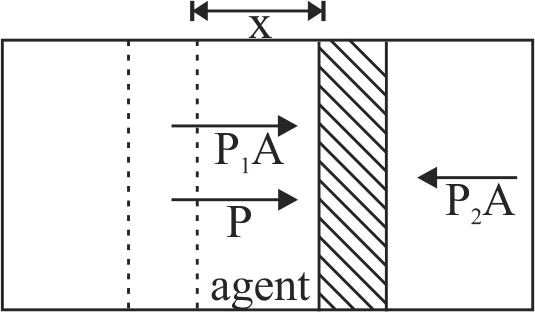
A piston can freely move inside a horizontal cylinder closed from both ends. Initially, the piston separates the inside space of the cylinder into two equal parts each of volume \(V_{0}\), in which an ideal gas is contained under the same pressure \(P_{0}\) and at the same temperature. What work has to be performed in order to increase isothermally the volume of one part of gas \(\eta\) times compared to that of the other by slowly moving the piston?
371625
The two conducting cylinder-piston systems shown below are linked. Cylinder 1 is filled with a certain molar quantity of a monatomic ideal gas, and cylinder 2 is filled with an equal molar quantity of a diatomic ideal gas. The entire apparatus is situated inside an oven whose temperature is \({T_{a}=27^{\circ} {C}}\). The cylinder volumes have the same initial value \({V_{0}=100 {cc}}\). When the oven temperature is slowly raised to \({T_{b}=127^{\circ} {C}}\). The volume change \({\Delta V}\) (in \(cc\)) of cylinder 1 is
371626
A horizontal frictionless piston, of negligible mass and heat capacity, divides a vertical insulated cylinder into two halves. Each half of the cylinder contains 1 mole of air at standard temperature and pressure \(p_{o}\). A load of weight \(W\) is now suspended from the piston, as shown in the figure. It pulls the piston down and comes to rest after a few oscillations. How large a volume does the compressed air in the lower part of the cylinder ultimately occupy if \(W\) is very large?
371627
A piston can freely move inside a horizontal cylinder closed from both ends. Initially, the piston separates the inside space of the cylinder into two equal parts each of volume \(V_{0}\), in which an ideal gas is contained under the same pressure \(P_{0}\) and at the same temperature. What work has to be performed in order to increase isothermally the volume of one part of gas \(\eta\) times compared to that of the other by slowly moving the piston?
371625
The two conducting cylinder-piston systems shown below are linked. Cylinder 1 is filled with a certain molar quantity of a monatomic ideal gas, and cylinder 2 is filled with an equal molar quantity of a diatomic ideal gas. The entire apparatus is situated inside an oven whose temperature is \({T_{a}=27^{\circ} {C}}\). The cylinder volumes have the same initial value \({V_{0}=100 {cc}}\). When the oven temperature is slowly raised to \({T_{b}=127^{\circ} {C}}\). The volume change \({\Delta V}\) (in \(cc\)) of cylinder 1 is
371626
A horizontal frictionless piston, of negligible mass and heat capacity, divides a vertical insulated cylinder into two halves. Each half of the cylinder contains 1 mole of air at standard temperature and pressure \(p_{o}\). A load of weight \(W\) is now suspended from the piston, as shown in the figure. It pulls the piston down and comes to rest after a few oscillations. How large a volume does the compressed air in the lower part of the cylinder ultimately occupy if \(W\) is very large?
371627
A piston can freely move inside a horizontal cylinder closed from both ends. Initially, the piston separates the inside space of the cylinder into two equal parts each of volume \(V_{0}\), in which an ideal gas is contained under the same pressure \(P_{0}\) and at the same temperature. What work has to be performed in order to increase isothermally the volume of one part of gas \(\eta\) times compared to that of the other by slowly moving the piston?
371625
The two conducting cylinder-piston systems shown below are linked. Cylinder 1 is filled with a certain molar quantity of a monatomic ideal gas, and cylinder 2 is filled with an equal molar quantity of a diatomic ideal gas. The entire apparatus is situated inside an oven whose temperature is \({T_{a}=27^{\circ} {C}}\). The cylinder volumes have the same initial value \({V_{0}=100 {cc}}\). When the oven temperature is slowly raised to \({T_{b}=127^{\circ} {C}}\). The volume change \({\Delta V}\) (in \(cc\)) of cylinder 1 is
371626
A horizontal frictionless piston, of negligible mass and heat capacity, divides a vertical insulated cylinder into two halves. Each half of the cylinder contains 1 mole of air at standard temperature and pressure \(p_{o}\). A load of weight \(W\) is now suspended from the piston, as shown in the figure. It pulls the piston down and comes to rest after a few oscillations. How large a volume does the compressed air in the lower part of the cylinder ultimately occupy if \(W\) is very large?
371627
A piston can freely move inside a horizontal cylinder closed from both ends. Initially, the piston separates the inside space of the cylinder into two equal parts each of volume \(V_{0}\), in which an ideal gas is contained under the same pressure \(P_{0}\) and at the same temperature. What work has to be performed in order to increase isothermally the volume of one part of gas \(\eta\) times compared to that of the other by slowly moving the piston?