371621
In the given figure, an adiabatic cylindrical tube of \(2 V_{o}\) is divided in two equal parts by a frictionless adiabatic separator. An ideal gas in left side of a tube having pressure \(P_{1}\) and temperature \(T_{1}\) where as in the right side having pressure \(P_{2}\) and temperature \(T_{2} . C_{p} / C_{v}=\gamma\) is the same for both the gases. The separator is slide slowly and is released at a position where it can stay in equilibrium. find the final volume of left part.
371622
An adiabatic cylindrical tube is fitted with an adiabatic separator as shown in figure. Initially separator is in equilibrium and divides a tube in two equal parts. The separator can be slide into the tube by an external mechanism. An ideal gas \((\gamma=1.5)\) is injected in the two sides at equal pressures and temperatures. Now separator is slide to a position where it divides the tube in the ratio \(7: 3\). Find the ratio of the temperatures in the two parts of the vessel.
371623
Two cylinders fitted with pistons and placed as shown, connected with string through a small tube of negligible volume, are filled with a gas at pressure \(p_{o}\) and temperature \(T_{o}\). The radius of smaller cylinder is half of the other. If the temperature is increased to \(2 T_{o}\), find the pressure, if the piston of bigger cylinder moves towards left by 1 metre?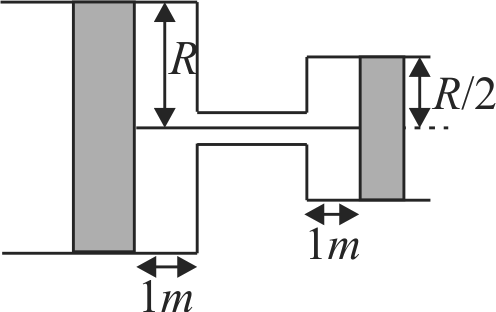
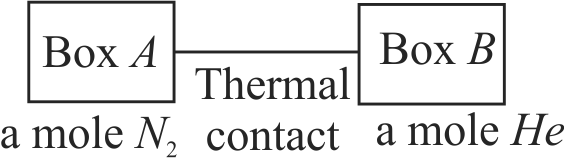
371624 Two rigid boxes containing different ideal gases are placed on table. Box \(A\) contains one mole of nitrogen at temperature \(T_{0}\), while box \(B\) contains one mole of helium at temperature \((7 / 3) T_{0}\). The boxes are then put into thermal contact with each other, and heat flows between them until the gases reach a common final temperature (ignore the heat capacity of boxes). Then, the final temperature of the gases \(T_{f}\) in terms of \(T_{0}\) is
371621
In the given figure, an adiabatic cylindrical tube of \(2 V_{o}\) is divided in two equal parts by a frictionless adiabatic separator. An ideal gas in left side of a tube having pressure \(P_{1}\) and temperature \(T_{1}\) where as in the right side having pressure \(P_{2}\) and temperature \(T_{2} . C_{p} / C_{v}=\gamma\) is the same for both the gases. The separator is slide slowly and is released at a position where it can stay in equilibrium. find the final volume of left part.
371622
An adiabatic cylindrical tube is fitted with an adiabatic separator as shown in figure. Initially separator is in equilibrium and divides a tube in two equal parts. The separator can be slide into the tube by an external mechanism. An ideal gas \((\gamma=1.5)\) is injected in the two sides at equal pressures and temperatures. Now separator is slide to a position where it divides the tube in the ratio \(7: 3\). Find the ratio of the temperatures in the two parts of the vessel.
371623
Two cylinders fitted with pistons and placed as shown, connected with string through a small tube of negligible volume, are filled with a gas at pressure \(p_{o}\) and temperature \(T_{o}\). The radius of smaller cylinder is half of the other. If the temperature is increased to \(2 T_{o}\), find the pressure, if the piston of bigger cylinder moves towards left by 1 metre?
371624 Two rigid boxes containing different ideal gases are placed on table. Box \(A\) contains one mole of nitrogen at temperature \(T_{0}\), while box \(B\) contains one mole of helium at temperature \((7 / 3) T_{0}\). The boxes are then put into thermal contact with each other, and heat flows between them until the gases reach a common final temperature (ignore the heat capacity of boxes). Then, the final temperature of the gases \(T_{f}\) in terms of \(T_{0}\) is
371621
In the given figure, an adiabatic cylindrical tube of \(2 V_{o}\) is divided in two equal parts by a frictionless adiabatic separator. An ideal gas in left side of a tube having pressure \(P_{1}\) and temperature \(T_{1}\) where as in the right side having pressure \(P_{2}\) and temperature \(T_{2} . C_{p} / C_{v}=\gamma\) is the same for both the gases. The separator is slide slowly and is released at a position where it can stay in equilibrium. find the final volume of left part.
371622
An adiabatic cylindrical tube is fitted with an adiabatic separator as shown in figure. Initially separator is in equilibrium and divides a tube in two equal parts. The separator can be slide into the tube by an external mechanism. An ideal gas \((\gamma=1.5)\) is injected in the two sides at equal pressures and temperatures. Now separator is slide to a position where it divides the tube in the ratio \(7: 3\). Find the ratio of the temperatures in the two parts of the vessel.
371623
Two cylinders fitted with pistons and placed as shown, connected with string through a small tube of negligible volume, are filled with a gas at pressure \(p_{o}\) and temperature \(T_{o}\). The radius of smaller cylinder is half of the other. If the temperature is increased to \(2 T_{o}\), find the pressure, if the piston of bigger cylinder moves towards left by 1 metre?
371624 Two rigid boxes containing different ideal gases are placed on table. Box \(A\) contains one mole of nitrogen at temperature \(T_{0}\), while box \(B\) contains one mole of helium at temperature \((7 / 3) T_{0}\). The boxes are then put into thermal contact with each other, and heat flows between them until the gases reach a common final temperature (ignore the heat capacity of boxes). Then, the final temperature of the gases \(T_{f}\) in terms of \(T_{0}\) is
371621
In the given figure, an adiabatic cylindrical tube of \(2 V_{o}\) is divided in two equal parts by a frictionless adiabatic separator. An ideal gas in left side of a tube having pressure \(P_{1}\) and temperature \(T_{1}\) where as in the right side having pressure \(P_{2}\) and temperature \(T_{2} . C_{p} / C_{v}=\gamma\) is the same for both the gases. The separator is slide slowly and is released at a position where it can stay in equilibrium. find the final volume of left part.
371622
An adiabatic cylindrical tube is fitted with an adiabatic separator as shown in figure. Initially separator is in equilibrium and divides a tube in two equal parts. The separator can be slide into the tube by an external mechanism. An ideal gas \((\gamma=1.5)\) is injected in the two sides at equal pressures and temperatures. Now separator is slide to a position where it divides the tube in the ratio \(7: 3\). Find the ratio of the temperatures in the two parts of the vessel.
371623
Two cylinders fitted with pistons and placed as shown, connected with string through a small tube of negligible volume, are filled with a gas at pressure \(p_{o}\) and temperature \(T_{o}\). The radius of smaller cylinder is half of the other. If the temperature is increased to \(2 T_{o}\), find the pressure, if the piston of bigger cylinder moves towards left by 1 metre?
371624 Two rigid boxes containing different ideal gases are placed on table. Box \(A\) contains one mole of nitrogen at temperature \(T_{0}\), while box \(B\) contains one mole of helium at temperature \((7 / 3) T_{0}\). The boxes are then put into thermal contact with each other, and heat flows between them until the gases reach a common final temperature (ignore the heat capacity of boxes). Then, the final temperature of the gases \(T_{f}\) in terms of \(T_{0}\) is