371517
There are two identical chambers, completely thermally insulated from surroundings. Both chambers have a partition wall dividing the chambers in two compartments. Compartment 1 is filled with an ideal gas and compartment 3 is filled with a real gas. Compartments 2 and 4 are vacuum. A small hole (orifice) is made in the partition walls and the gases are allowed to expand in vacuum.
Statement A :
No change in the temperature of the gas takes place when ideal gas expands in vacuum. However, the temperature of real gas goes down (cooling) when it expands in vacuum.
Statement B :
The internal energy of an ideal gas is only kinetic. The internal energy of a real gas is kinetic as well as potential.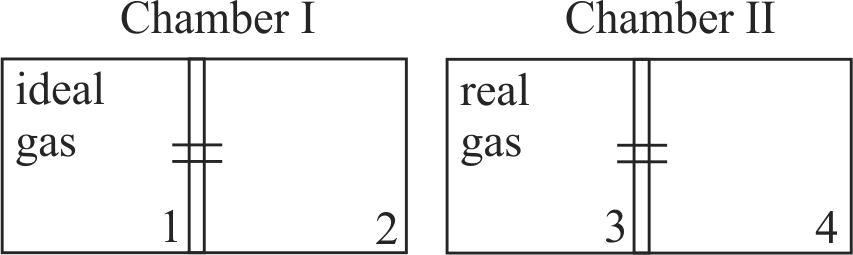
371520
An ideal gas at \(NTP\) is enclosed in an adiabatic vertical cylinder having an area of cross section \(A = 27\;c{m^2}\) between two light movable piston as shown in the fig. Spring with force constant \(k = 3700\;N/m\) is in a relaxed state initially. Now the lower piston is moved upwards a distance \(h / 2\), \(h\) being the initial length of gas column. It is observed that the upper piston moves up by a distance \(h / 16\). Final temperature of gas \( = 4/3 \times 273\,K\). Take \(\gamma\) for the gas to be \(3 / 2\). The value of \(h\) is: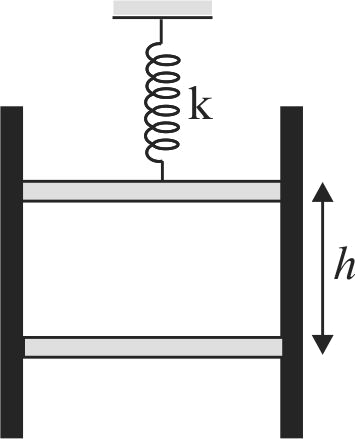
371517
There are two identical chambers, completely thermally insulated from surroundings. Both chambers have a partition wall dividing the chambers in two compartments. Compartment 1 is filled with an ideal gas and compartment 3 is filled with a real gas. Compartments 2 and 4 are vacuum. A small hole (orifice) is made in the partition walls and the gases are allowed to expand in vacuum.
Statement A :
No change in the temperature of the gas takes place when ideal gas expands in vacuum. However, the temperature of real gas goes down (cooling) when it expands in vacuum.
Statement B :
The internal energy of an ideal gas is only kinetic. The internal energy of a real gas is kinetic as well as potential.
371520
An ideal gas at \(NTP\) is enclosed in an adiabatic vertical cylinder having an area of cross section \(A = 27\;c{m^2}\) between two light movable piston as shown in the fig. Spring with force constant \(k = 3700\;N/m\) is in a relaxed state initially. Now the lower piston is moved upwards a distance \(h / 2\), \(h\) being the initial length of gas column. It is observed that the upper piston moves up by a distance \(h / 16\). Final temperature of gas \( = 4/3 \times 273\,K\). Take \(\gamma\) for the gas to be \(3 / 2\). The value of \(h\) is:
371517
There are two identical chambers, completely thermally insulated from surroundings. Both chambers have a partition wall dividing the chambers in two compartments. Compartment 1 is filled with an ideal gas and compartment 3 is filled with a real gas. Compartments 2 and 4 are vacuum. A small hole (orifice) is made in the partition walls and the gases are allowed to expand in vacuum.
Statement A :
No change in the temperature of the gas takes place when ideal gas expands in vacuum. However, the temperature of real gas goes down (cooling) when it expands in vacuum.
Statement B :
The internal energy of an ideal gas is only kinetic. The internal energy of a real gas is kinetic as well as potential.
371520
An ideal gas at \(NTP\) is enclosed in an adiabatic vertical cylinder having an area of cross section \(A = 27\;c{m^2}\) between two light movable piston as shown in the fig. Spring with force constant \(k = 3700\;N/m\) is in a relaxed state initially. Now the lower piston is moved upwards a distance \(h / 2\), \(h\) being the initial length of gas column. It is observed that the upper piston moves up by a distance \(h / 16\). Final temperature of gas \( = 4/3 \times 273\,K\). Take \(\gamma\) for the gas to be \(3 / 2\). The value of \(h\) is:
371517
There are two identical chambers, completely thermally insulated from surroundings. Both chambers have a partition wall dividing the chambers in two compartments. Compartment 1 is filled with an ideal gas and compartment 3 is filled with a real gas. Compartments 2 and 4 are vacuum. A small hole (orifice) is made in the partition walls and the gases are allowed to expand in vacuum.
Statement A :
No change in the temperature of the gas takes place when ideal gas expands in vacuum. However, the temperature of real gas goes down (cooling) when it expands in vacuum.
Statement B :
The internal energy of an ideal gas is only kinetic. The internal energy of a real gas is kinetic as well as potential.
371520
An ideal gas at \(NTP\) is enclosed in an adiabatic vertical cylinder having an area of cross section \(A = 27\;c{m^2}\) between two light movable piston as shown in the fig. Spring with force constant \(k = 3700\;N/m\) is in a relaxed state initially. Now the lower piston is moved upwards a distance \(h / 2\), \(h\) being the initial length of gas column. It is observed that the upper piston moves up by a distance \(h / 16\). Final temperature of gas \( = 4/3 \times 273\,K\). Take \(\gamma\) for the gas to be \(3 / 2\). The value of \(h\) is:
