371341
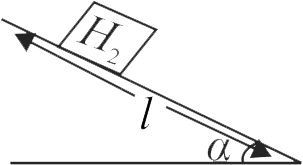
Rigid vessel of negligible mass containing \(3\) moles of hydrogen gas is set free from a rough inclined plane of length \(\sqrt 3 \;km\) and angle of inclination \(60^{\circ}\). One third of heat due to friction is absorbed by the hydrogen gas. If the vessel stops just before reaching the bottom of the incline, then change in temperature of the hydrogen gas is
(Take \(R = 8.0\;J\;mo{l^{ - 1}}\;{K^{ - 1}},{C_{di}} = \frac{5}{2}R\))
371342
Match the Column I (quantity) with Column II (value) and select the correct answer from the codes given below.
Column I
Column II
A
\(1\,\,cal = \)
P
\(4.186\;J/gK\)
B
\(p{\left( {\frac{{\Delta V}}{{\Delta T}}} \right)_p} = \)
Q
\(nR\)
C
Specific heat capacity of solids
R
\(\Delta Q\)
D
For isothermal process, \(W\)
S
\(3R\)
371343
One of the straight lines in the Fig. depicts the dependence of the work done \(|W|\) on the temperature variations \(\mathrm{T}\) for an isobaric process. The two of following are the adiabatic curves for argon and nitrogen. The isobaric process which depicts both the gases is: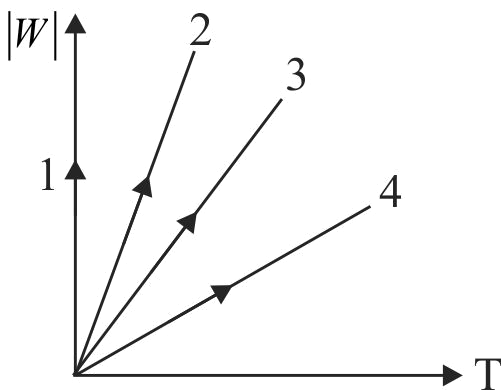
371344
Choose the incorrect statement from the following.
S1 : The efficiency of a heat engine can be 1 , but the coefficient of performance of a refrigerator can never be infinity.
S2 : The first law of thermodynamics is basically the principle of conservation of energy.
S3 : The second law of thermodynamics does not allow several phenomena consistent with the first law.
S4 : A process, whose sole result is the transfer of heat from a colder object to a hotter object is impossible.
371341
Rigid vessel of negligible mass containing \(3\) moles of hydrogen gas is set free from a rough inclined plane of length \(\sqrt 3 \;km\) and angle of inclination \(60^{\circ}\). One third of heat due to friction is absorbed by the hydrogen gas. If the vessel stops just before reaching the bottom of the incline, then change in temperature of the hydrogen gas is
(Take \(R = 8.0\;J\;mo{l^{ - 1}}\;{K^{ - 1}},{C_{di}} = \frac{5}{2}R\))
371342
Match the Column I (quantity) with Column II (value) and select the correct answer from the codes given below.
Column I
Column II
A
\(1\,\,cal = \)
P
\(4.186\;J/gK\)
B
\(p{\left( {\frac{{\Delta V}}{{\Delta T}}} \right)_p} = \)
Q
\(nR\)
C
Specific heat capacity of solids
R
\(\Delta Q\)
D
For isothermal process, \(W\)
S
\(3R\)
371343
One of the straight lines in the Fig. depicts the dependence of the work done \(|W|\) on the temperature variations \(\mathrm{T}\) for an isobaric process. The two of following are the adiabatic curves for argon and nitrogen. The isobaric process which depicts both the gases is:
371344
Choose the incorrect statement from the following.
S1 : The efficiency of a heat engine can be 1 , but the coefficient of performance of a refrigerator can never be infinity.
S2 : The first law of thermodynamics is basically the principle of conservation of energy.
S3 : The second law of thermodynamics does not allow several phenomena consistent with the first law.
S4 : A process, whose sole result is the transfer of heat from a colder object to a hotter object is impossible.
371341
Rigid vessel of negligible mass containing \(3\) moles of hydrogen gas is set free from a rough inclined plane of length \(\sqrt 3 \;km\) and angle of inclination \(60^{\circ}\). One third of heat due to friction is absorbed by the hydrogen gas. If the vessel stops just before reaching the bottom of the incline, then change in temperature of the hydrogen gas is
(Take \(R = 8.0\;J\;mo{l^{ - 1}}\;{K^{ - 1}},{C_{di}} = \frac{5}{2}R\))
371342
Match the Column I (quantity) with Column II (value) and select the correct answer from the codes given below.
Column I
Column II
A
\(1\,\,cal = \)
P
\(4.186\;J/gK\)
B
\(p{\left( {\frac{{\Delta V}}{{\Delta T}}} \right)_p} = \)
Q
\(nR\)
C
Specific heat capacity of solids
R
\(\Delta Q\)
D
For isothermal process, \(W\)
S
\(3R\)
371343
One of the straight lines in the Fig. depicts the dependence of the work done \(|W|\) on the temperature variations \(\mathrm{T}\) for an isobaric process. The two of following are the adiabatic curves for argon and nitrogen. The isobaric process which depicts both the gases is:
371344
Choose the incorrect statement from the following.
S1 : The efficiency of a heat engine can be 1 , but the coefficient of performance of a refrigerator can never be infinity.
S2 : The first law of thermodynamics is basically the principle of conservation of energy.
S3 : The second law of thermodynamics does not allow several phenomena consistent with the first law.
S4 : A process, whose sole result is the transfer of heat from a colder object to a hotter object is impossible.
371341
Rigid vessel of negligible mass containing \(3\) moles of hydrogen gas is set free from a rough inclined plane of length \(\sqrt 3 \;km\) and angle of inclination \(60^{\circ}\). One third of heat due to friction is absorbed by the hydrogen gas. If the vessel stops just before reaching the bottom of the incline, then change in temperature of the hydrogen gas is
(Take \(R = 8.0\;J\;mo{l^{ - 1}}\;{K^{ - 1}},{C_{di}} = \frac{5}{2}R\))
371342
Match the Column I (quantity) with Column II (value) and select the correct answer from the codes given below.
Column I
Column II
A
\(1\,\,cal = \)
P
\(4.186\;J/gK\)
B
\(p{\left( {\frac{{\Delta V}}{{\Delta T}}} \right)_p} = \)
Q
\(nR\)
C
Specific heat capacity of solids
R
\(\Delta Q\)
D
For isothermal process, \(W\)
S
\(3R\)
371343
One of the straight lines in the Fig. depicts the dependence of the work done \(|W|\) on the temperature variations \(\mathrm{T}\) for an isobaric process. The two of following are the adiabatic curves for argon and nitrogen. The isobaric process which depicts both the gases is:
371344
Choose the incorrect statement from the following.
S1 : The efficiency of a heat engine can be 1 , but the coefficient of performance of a refrigerator can never be infinity.
S2 : The first law of thermodynamics is basically the principle of conservation of energy.
S3 : The second law of thermodynamics does not allow several phenomena consistent with the first law.
S4 : A process, whose sole result is the transfer of heat from a colder object to a hotter object is impossible.