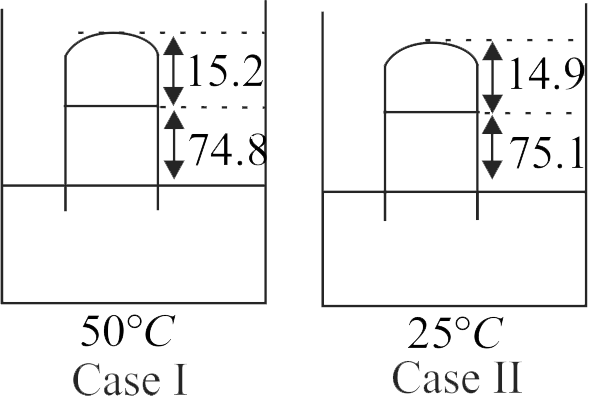
371339 A barometer tube has length \(90\;cm.\) It contains some air above mercury. On some hot days when temperature is \(50^\circ C\) and true atmospheric pressure is \(75\;cm\) of \(Hg,\) the reading of the mercury level is \(74.8\;cm.\) If the reading is observed to be \(75.1\;cm\) on some another day when temperature is \(25^\circ C,\) then Find the true pressure on that day will be
371339 A barometer tube has length \(90\;cm.\) It contains some air above mercury. On some hot days when temperature is \(50^\circ C\) and true atmospheric pressure is \(75\;cm\) of \(Hg,\) the reading of the mercury level is \(74.8\;cm.\) If the reading is observed to be \(75.1\;cm\) on some another day when temperature is \(25^\circ C,\) then Find the true pressure on that day will be
371339 A barometer tube has length \(90\;cm.\) It contains some air above mercury. On some hot days when temperature is \(50^\circ C\) and true atmospheric pressure is \(75\;cm\) of \(Hg,\) the reading of the mercury level is \(74.8\;cm.\) If the reading is observed to be \(75.1\;cm\) on some another day when temperature is \(25^\circ C,\) then Find the true pressure on that day will be
371339 A barometer tube has length \(90\;cm.\) It contains some air above mercury. On some hot days when temperature is \(50^\circ C\) and true atmospheric pressure is \(75\;cm\) of \(Hg,\) the reading of the mercury level is \(74.8\;cm.\) If the reading is observed to be \(75.1\;cm\) on some another day when temperature is \(25^\circ C,\) then Find the true pressure on that day will be