371264
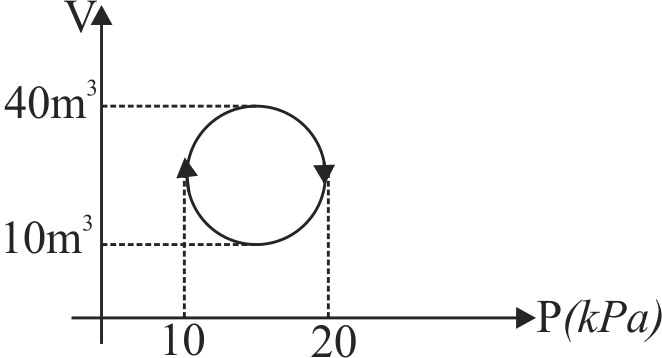
An ideal gas after going through a series of four thermodynamic states in order, reaches the initial state against (cyclic process). The amounts of heat and work involved in these states are
The ratio of net work done by the gas to the total heat absorbed by the gas is
371264
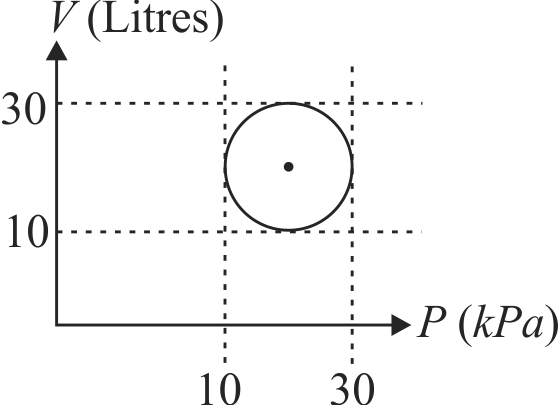
An ideal gas after going through a series of four thermodynamic states in order, reaches the initial state against (cyclic process). The amounts of heat and work involved in these states are
The ratio of net work done by the gas to the total heat absorbed by the gas is
371264
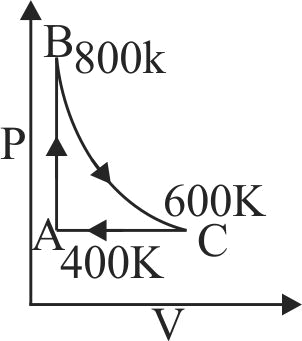
An ideal gas after going through a series of four thermodynamic states in order, reaches the initial state against (cyclic process). The amounts of heat and work involved in these states are
The ratio of net work done by the gas to the total heat absorbed by the gas is
371264
An ideal gas after going through a series of four thermodynamic states in order, reaches the initial state against (cyclic process). The amounts of heat and work involved in these states are
The ratio of net work done by the gas to the total heat absorbed by the gas is