371258
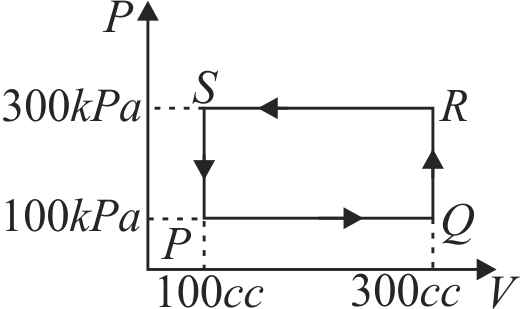
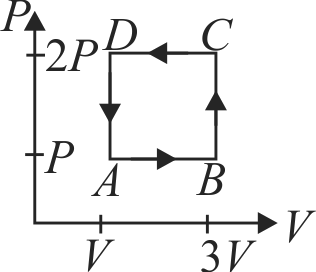
A gas can be taken from \(A\) to \(B\) via two different processes \(A C B\) and \(A D B\). When path \(A C B\) is used \(60\;J\) of heat flows into the system and \(30\,J\) of work is done by the system. If path \(A D B\) is used work done by the system is \(10\,J\). The heat flow into the system in path \(A D B\) is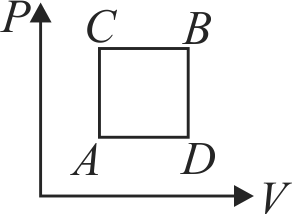
371259
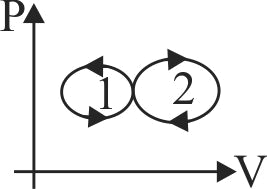
A cyclic Process of an ideal gas is shown in the figure. Let \(W_{a \rightarrow b}, W_{b \rightarrow c}, W_{c \rightarrow d}\) and \(W_{d \rightarrow a}\)
represents the work done by the system during the processes \(a \rightarrow b, b \rightarrow c, c \rightarrow d\) and \(d \rightarrow a\) respectively. Consider the following relations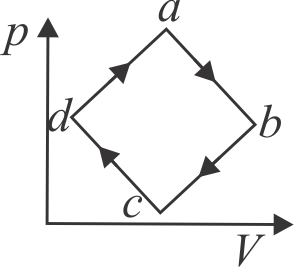
I. \(W_{a \rightarrow b}+W_{b \rightarrow c}+W_{c \rightarrow d}+W_{d \rightarrow a}>0\)
II. \(W_{a \rightarrow b}+W_{b \rightarrow c}+W_{c \rightarrow a}+W_{d \rightarrow a} < 0\)
III. \(W_{a \rightarrow b}+W_{c \rightarrow d}>0\)
IV. \(W_{b \rightarrow c}+W_{d \rightarrow a}=0 \quad\)
Which of the above relations is/are true?
371258
A gas can be taken from \(A\) to \(B\) via two different processes \(A C B\) and \(A D B\). When path \(A C B\) is used \(60\;J\) of heat flows into the system and \(30\,J\) of work is done by the system. If path \(A D B\) is used work done by the system is \(10\,J\). The heat flow into the system in path \(A D B\) is
371259
A cyclic Process of an ideal gas is shown in the figure. Let \(W_{a \rightarrow b}, W_{b \rightarrow c}, W_{c \rightarrow d}\) and \(W_{d \rightarrow a}\)
represents the work done by the system during the processes \(a \rightarrow b, b \rightarrow c, c \rightarrow d\) and \(d \rightarrow a\) respectively. Consider the following relations
I. \(W_{a \rightarrow b}+W_{b \rightarrow c}+W_{c \rightarrow d}+W_{d \rightarrow a}>0\)
II. \(W_{a \rightarrow b}+W_{b \rightarrow c}+W_{c \rightarrow a}+W_{d \rightarrow a} < 0\)
III. \(W_{a \rightarrow b}+W_{c \rightarrow d}>0\)
IV. \(W_{b \rightarrow c}+W_{d \rightarrow a}=0 \quad\)
Which of the above relations is/are true?
371258
A gas can be taken from \(A\) to \(B\) via two different processes \(A C B\) and \(A D B\). When path \(A C B\) is used \(60\;J\) of heat flows into the system and \(30\,J\) of work is done by the system. If path \(A D B\) is used work done by the system is \(10\,J\). The heat flow into the system in path \(A D B\) is
371259
A cyclic Process of an ideal gas is shown in the figure. Let \(W_{a \rightarrow b}, W_{b \rightarrow c}, W_{c \rightarrow d}\) and \(W_{d \rightarrow a}\)
represents the work done by the system during the processes \(a \rightarrow b, b \rightarrow c, c \rightarrow d\) and \(d \rightarrow a\) respectively. Consider the following relations
I. \(W_{a \rightarrow b}+W_{b \rightarrow c}+W_{c \rightarrow d}+W_{d \rightarrow a}>0\)
II. \(W_{a \rightarrow b}+W_{b \rightarrow c}+W_{c \rightarrow a}+W_{d \rightarrow a} < 0\)
III. \(W_{a \rightarrow b}+W_{c \rightarrow d}>0\)
IV. \(W_{b \rightarrow c}+W_{d \rightarrow a}=0 \quad\)
Which of the above relations is/are true?
371258
A gas can be taken from \(A\) to \(B\) via two different processes \(A C B\) and \(A D B\). When path \(A C B\) is used \(60\;J\) of heat flows into the system and \(30\,J\) of work is done by the system. If path \(A D B\) is used work done by the system is \(10\,J\). The heat flow into the system in path \(A D B\) is
371259
A cyclic Process of an ideal gas is shown in the figure. Let \(W_{a \rightarrow b}, W_{b \rightarrow c}, W_{c \rightarrow d}\) and \(W_{d \rightarrow a}\)
represents the work done by the system during the processes \(a \rightarrow b, b \rightarrow c, c \rightarrow d\) and \(d \rightarrow a\) respectively. Consider the following relations
I. \(W_{a \rightarrow b}+W_{b \rightarrow c}+W_{c \rightarrow d}+W_{d \rightarrow a}>0\)
II. \(W_{a \rightarrow b}+W_{b \rightarrow c}+W_{c \rightarrow a}+W_{d \rightarrow a} < 0\)
III. \(W_{a \rightarrow b}+W_{c \rightarrow d}>0\)
IV. \(W_{b \rightarrow c}+W_{d \rightarrow a}=0 \quad\)
Which of the above relations is/are true?
371258
A gas can be taken from \(A\) to \(B\) via two different processes \(A C B\) and \(A D B\). When path \(A C B\) is used \(60\;J\) of heat flows into the system and \(30\,J\) of work is done by the system. If path \(A D B\) is used work done by the system is \(10\,J\). The heat flow into the system in path \(A D B\) is
371259
A cyclic Process of an ideal gas is shown in the figure. Let \(W_{a \rightarrow b}, W_{b \rightarrow c}, W_{c \rightarrow d}\) and \(W_{d \rightarrow a}\)
represents the work done by the system during the processes \(a \rightarrow b, b \rightarrow c, c \rightarrow d\) and \(d \rightarrow a\) respectively. Consider the following relations
I. \(W_{a \rightarrow b}+W_{b \rightarrow c}+W_{c \rightarrow d}+W_{d \rightarrow a}>0\)
II. \(W_{a \rightarrow b}+W_{b \rightarrow c}+W_{c \rightarrow a}+W_{d \rightarrow a} < 0\)
III. \(W_{a \rightarrow b}+W_{c \rightarrow d}>0\)
IV. \(W_{b \rightarrow c}+W_{d \rightarrow a}=0 \quad\)
Which of the above relations is/are true?