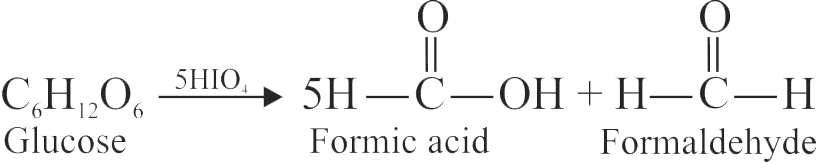
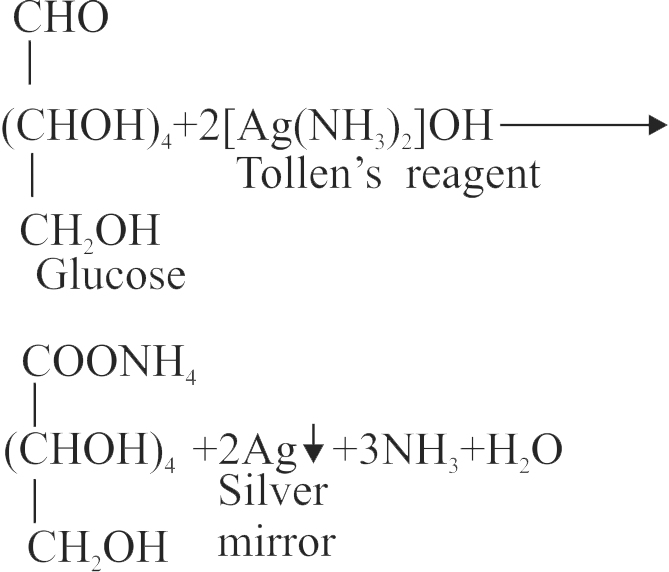
324625
Consider the statements
I. The \(\mathrm{[\alpha]_{D}}\) of sucrose is \({\rm{ + 66}}{\rm{.5}}^\circ \)
II. The \(\mathrm{[\alpha]_{D}}\) of \(\mathrm{\alpha}\)-D-Glucose is \({\rm{ + 52}}{\rm{.5}}^\circ \)
III. The \(\mathrm{[\alpha]_{D}}\) of \(\mathrm{\beta}\)-D-Fructose is \({\rm{ + 92}}{\rm{.4}}^\circ \)
The correct statements is/are
324625
Consider the statements
I. The \(\mathrm{[\alpha]_{D}}\) of sucrose is \({\rm{ + 66}}{\rm{.5}}^\circ \)
II. The \(\mathrm{[\alpha]_{D}}\) of \(\mathrm{\alpha}\)-D-Glucose is \({\rm{ + 52}}{\rm{.5}}^\circ \)
III. The \(\mathrm{[\alpha]_{D}}\) of \(\mathrm{\beta}\)-D-Fructose is \({\rm{ + 92}}{\rm{.4}}^\circ \)
The correct statements is/are
324625
Consider the statements
I. The \(\mathrm{[\alpha]_{D}}\) of sucrose is \({\rm{ + 66}}{\rm{.5}}^\circ \)
II. The \(\mathrm{[\alpha]_{D}}\) of \(\mathrm{\alpha}\)-D-Glucose is \({\rm{ + 52}}{\rm{.5}}^\circ \)
III. The \(\mathrm{[\alpha]_{D}}\) of \(\mathrm{\beta}\)-D-Fructose is \({\rm{ + 92}}{\rm{.4}}^\circ \)
The correct statements is/are
324625
Consider the statements
I. The \(\mathrm{[\alpha]_{D}}\) of sucrose is \({\rm{ + 66}}{\rm{.5}}^\circ \)
II. The \(\mathrm{[\alpha]_{D}}\) of \(\mathrm{\alpha}\)-D-Glucose is \({\rm{ + 52}}{\rm{.5}}^\circ \)
III. The \(\mathrm{[\alpha]_{D}}\) of \(\mathrm{\beta}\)-D-Fructose is \({\rm{ + 92}}{\rm{.4}}^\circ \)
The correct statements is/are
324625
Consider the statements
I. The \(\mathrm{[\alpha]_{D}}\) of sucrose is \({\rm{ + 66}}{\rm{.5}}^\circ \)
II. The \(\mathrm{[\alpha]_{D}}\) of \(\mathrm{\alpha}\)-D-Glucose is \({\rm{ + 52}}{\rm{.5}}^\circ \)
III. The \(\mathrm{[\alpha]_{D}}\) of \(\mathrm{\beta}\)-D-Fructose is \({\rm{ + 92}}{\rm{.4}}^\circ \)
The correct statements is/are