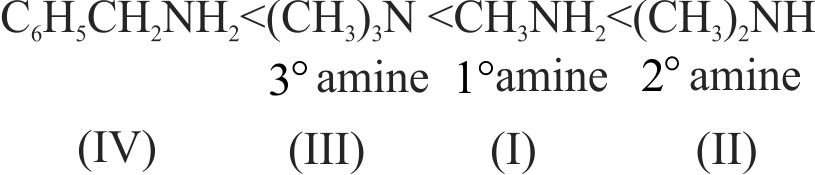324094 Arrange the following in the increasing order of their basic strength.\(\mathrm{CH}_{3} \mathrm{NH}_{2}\) (I);\(\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}\) (II);\(\left(\mathrm{CH}_{3}\right)_{3} \mathrm{~N}(\mathrm{III})\);\(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}\) (IV)
324094 Arrange the following in the increasing order of their basic strength.\(\mathrm{CH}_{3} \mathrm{NH}_{2}\) (I);\(\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}\) (II);\(\left(\mathrm{CH}_{3}\right)_{3} \mathrm{~N}(\mathrm{III})\);\(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}\) (IV)
324094 Arrange the following in the increasing order of their basic strength.\(\mathrm{CH}_{3} \mathrm{NH}_{2}\) (I);\(\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}\) (II);\(\left(\mathrm{CH}_{3}\right)_{3} \mathrm{~N}(\mathrm{III})\);\(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}\) (IV)
324094 Arrange the following in the increasing order of their basic strength.\(\mathrm{CH}_{3} \mathrm{NH}_{2}\) (I);\(\left(\mathrm{CH}_{3}\right)_{2} \mathrm{NH}\) (II);\(\left(\mathrm{CH}_{3}\right)_{3} \mathrm{~N}(\mathrm{III})\);\(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{NH}_{2}\) (IV)