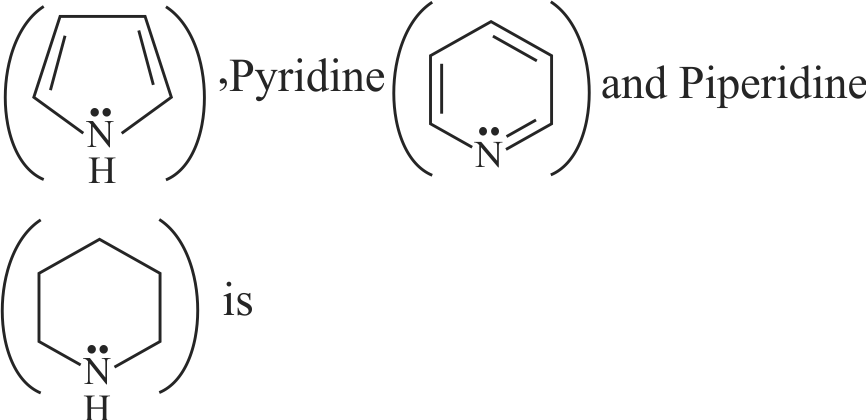
324060
Assertion :
\(\mathrm{Me}_{3} \mathrm{~N}\) reacts with \(\mathrm{BF}_{3}\) whereas \(\mathrm{Ph}_{3} \mathrm{~N}\) does not.
Reason :
The electron pair on nitrogen atom in \({\text{N}}{{\text{H}}_{\text{3}}}\) is delocalised in the benzene ring and is not available to boron in \(\mathrm{BF}_{3}\).
324044
Assertion :
Basicity of \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{NH}_{2}\) (I), \(\mathrm{NH}_{3}\) (II) and \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}\) (III) is in the order I \(>\) II \(>\) III.
Reason :
Electron-donating groups (such as alkyl group) increase the basicity of amines, and electron-withdrawing groups (such as aryl group) decrease the basicity of amines.
324060
Assertion :
\(\mathrm{Me}_{3} \mathrm{~N}\) reacts with \(\mathrm{BF}_{3}\) whereas \(\mathrm{Ph}_{3} \mathrm{~N}\) does not.
Reason :
The electron pair on nitrogen atom in \({\text{N}}{{\text{H}}_{\text{3}}}\) is delocalised in the benzene ring and is not available to boron in \(\mathrm{BF}_{3}\).
324044
Assertion :
Basicity of \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{NH}_{2}\) (I), \(\mathrm{NH}_{3}\) (II) and \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}\) (III) is in the order I \(>\) II \(>\) III.
Reason :
Electron-donating groups (such as alkyl group) increase the basicity of amines, and electron-withdrawing groups (such as aryl group) decrease the basicity of amines.
324060
Assertion :
\(\mathrm{Me}_{3} \mathrm{~N}\) reacts with \(\mathrm{BF}_{3}\) whereas \(\mathrm{Ph}_{3} \mathrm{~N}\) does not.
Reason :
The electron pair on nitrogen atom in \({\text{N}}{{\text{H}}_{\text{3}}}\) is delocalised in the benzene ring and is not available to boron in \(\mathrm{BF}_{3}\).
324044
Assertion :
Basicity of \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{NH}_{2}\) (I), \(\mathrm{NH}_{3}\) (II) and \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}\) (III) is in the order I \(>\) II \(>\) III.
Reason :
Electron-donating groups (such as alkyl group) increase the basicity of amines, and electron-withdrawing groups (such as aryl group) decrease the basicity of amines.
324060
Assertion :
\(\mathrm{Me}_{3} \mathrm{~N}\) reacts with \(\mathrm{BF}_{3}\) whereas \(\mathrm{Ph}_{3} \mathrm{~N}\) does not.
Reason :
The electron pair on nitrogen atom in \({\text{N}}{{\text{H}}_{\text{3}}}\) is delocalised in the benzene ring and is not available to boron in \(\mathrm{BF}_{3}\).
324044
Assertion :
Basicity of \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{NH}_{2}\) (I), \(\mathrm{NH}_{3}\) (II) and \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{NH}_{2}\) (III) is in the order I \(>\) II \(>\) III.
Reason :
Electron-donating groups (such as alkyl group) increase the basicity of amines, and electron-withdrawing groups (such as aryl group) decrease the basicity of amines.