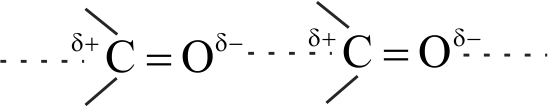323941
Arrange the following compounds in decreasing order of their boiling points.
\({\text{C}}{{\text{H}}_3}{\text{CHO}},{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}},{\text{C}}{{\text{H}}_3}{\text{OC}}{{\text{H}}_3},\)\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\)
323936
Arrange the following compounds in the increasing order of their boiling points.
\(\begin{array}{*{20}{c}}{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{CHO(I)}}}&{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{OH}}\,\,{\rm{(II)}}}\\{{{\rm{H}}_{\rm{5}}}{{\rm{C}}_{\rm{2}}}{\rm{O}}{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}\,\,{\rm{(III)}}}&{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{(IV)}}}\end{array}\)
323937
Given below are two statements :-
Statement I :
The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions.
Statements II :
The boiling points aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding.
In the light of the statements, choose the most appropriate answer from the options given below
323938
Statement A :
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses.
Statement B :
There is a weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
323941
Arrange the following compounds in decreasing order of their boiling points.
\({\text{C}}{{\text{H}}_3}{\text{CHO}},{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}},{\text{C}}{{\text{H}}_3}{\text{OC}}{{\text{H}}_3},\)\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\)
323936
Arrange the following compounds in the increasing order of their boiling points.
\(\begin{array}{*{20}{c}}{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{CHO(I)}}}&{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{OH}}\,\,{\rm{(II)}}}\\{{{\rm{H}}_{\rm{5}}}{{\rm{C}}_{\rm{2}}}{\rm{O}}{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}\,\,{\rm{(III)}}}&{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{(IV)}}}\end{array}\)
323937
Given below are two statements :-
Statement I :
The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions.
Statements II :
The boiling points aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding.
In the light of the statements, choose the most appropriate answer from the options given below
323938
Statement A :
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses.
Statement B :
There is a weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
323941
Arrange the following compounds in decreasing order of their boiling points.
\({\text{C}}{{\text{H}}_3}{\text{CHO}},{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}},{\text{C}}{{\text{H}}_3}{\text{OC}}{{\text{H}}_3},\)\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\)
323936
Arrange the following compounds in the increasing order of their boiling points.
\(\begin{array}{*{20}{c}}{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{CHO(I)}}}&{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{OH}}\,\,{\rm{(II)}}}\\{{{\rm{H}}_{\rm{5}}}{{\rm{C}}_{\rm{2}}}{\rm{O}}{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}\,\,{\rm{(III)}}}&{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{(IV)}}}\end{array}\)
323937
Given below are two statements :-
Statement I :
The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions.
Statements II :
The boiling points aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding.
In the light of the statements, choose the most appropriate answer from the options given below
323938
Statement A :
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses.
Statement B :
There is a weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
323941
Arrange the following compounds in decreasing order of their boiling points.
\({\text{C}}{{\text{H}}_3}{\text{CHO}},{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}},{\text{C}}{{\text{H}}_3}{\text{OC}}{{\text{H}}_3},\)\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\)
323936
Arrange the following compounds in the increasing order of their boiling points.
\(\begin{array}{*{20}{c}}{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{CHO(I)}}}&{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{OH}}\,\,{\rm{(II)}}}\\{{{\rm{H}}_{\rm{5}}}{{\rm{C}}_{\rm{2}}}{\rm{O}}{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}\,\,{\rm{(III)}}}&{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{(IV)}}}\end{array}\)
323937
Given below are two statements :-
Statement I :
The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions.
Statements II :
The boiling points aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding.
In the light of the statements, choose the most appropriate answer from the options given below
323938
Statement A :
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses.
Statement B :
There is a weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
323941
Arrange the following compounds in decreasing order of their boiling points.
\({\text{C}}{{\text{H}}_3}{\text{CHO}},{\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}},{\text{C}}{{\text{H}}_3}{\text{OC}}{{\text{H}}_3},\)\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{C}}{{\text{H}}_3}\)
323936
Arrange the following compounds in the increasing order of their boiling points.
\(\begin{array}{*{20}{c}}{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{CHO(I)}}}&{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{OH}}\,\,{\rm{(II)}}}\\{{{\rm{H}}_{\rm{5}}}{{\rm{C}}_{\rm{2}}}{\rm{O}}{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{5}}}\,\,{\rm{(III)}}}&{{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{2}}}{\rm{C}}{{\rm{H}}_{\rm{3}}}{\rm{(IV)}}}\end{array}\)
323937
Given below are two statements :-
Statement I :
The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions.
Statements II :
The boiling points aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding.
In the light of the statements, choose the most appropriate answer from the options given below
323938
Statement A :
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses.
Statement B :
There is a weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.