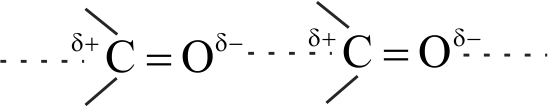323937
Given below are two statements :-
Statement I :
The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions.
Statements II :
The boiling points aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding.
In the light of the statements, choose the most appropriate answer from the options given below
323938
Statement A :
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses.
Statement B :
There is a weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
323937
Given below are two statements :-
Statement I :
The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions.
Statements II :
The boiling points aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding.
In the light of the statements, choose the most appropriate answer from the options given below
323938
Statement A :
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses.
Statement B :
There is a weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
323937
Given below are two statements :-
Statement I :
The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions.
Statements II :
The boiling points aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding.
In the light of the statements, choose the most appropriate answer from the options given below
323938
Statement A :
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses.
Statement B :
There is a weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
323937
Given below are two statements :-
Statement I :
The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions.
Statements II :
The boiling points aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding.
In the light of the statements, choose the most appropriate answer from the options given below
323938
Statement A :
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses.
Statement B :
There is a weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.
323937
Given below are two statements :-
Statement I :
The boiling points of aldehydes and ketones are higher than hydrocarbons of comparable molecular masses because of weak molecular association in aldehydes and ketones due to dipole - dipole interactions.
Statements II :
The boiling points aldehydes and ketones are lower than the alcohols of similar molecular masses due to the absence of H-bonding.
In the light of the statements, choose the most appropriate answer from the options given below
323938
Statement A :
The boiling points of aldehydes and ketones are higher than hydrocarbons and ethers of comparable molecular masses.
Statement B :
There is a weak molecular association in aldehydes and ketones arising out of the dipole-dipole interactions.