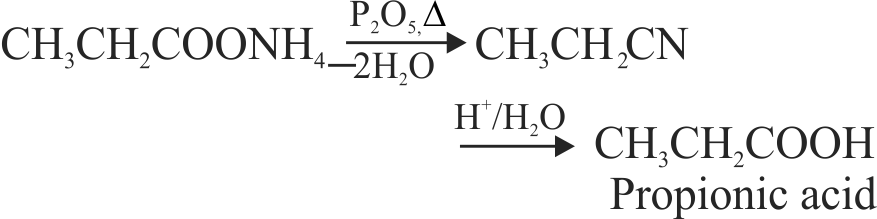
323888 In the reaction, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{4}}}\xrightarrow{{{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}}}{\text{A}}\xrightarrow{{{{\text{H}}^{\text{ + }}}{\text{/}}{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{B}}\) A and B are
323890
Which of the following can't form \(\mathrm{CH}_{3} \mathrm{COOH}\) from \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) ?
\({\text{(A) PCC}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(B)PDC}}\)
\({\text{(C) }}{{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{/}}{{\text{H}}^{\text{ + }}}\,\,\,\,\,\,\,\,\,{\text{(D)Micoderma}}\,\,{\text{aceti}}\)
323888 In the reaction, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{4}}}\xrightarrow{{{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}}}{\text{A}}\xrightarrow{{{{\text{H}}^{\text{ + }}}{\text{/}}{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{B}}\) A and B are
323890
Which of the following can't form \(\mathrm{CH}_{3} \mathrm{COOH}\) from \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) ?
\({\text{(A) PCC}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(B)PDC}}\)
\({\text{(C) }}{{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{/}}{{\text{H}}^{\text{ + }}}\,\,\,\,\,\,\,\,\,{\text{(D)Micoderma}}\,\,{\text{aceti}}\)
323888 In the reaction, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{4}}}\xrightarrow{{{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}}}{\text{A}}\xrightarrow{{{{\text{H}}^{\text{ + }}}{\text{/}}{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{B}}\) A and B are
323890
Which of the following can't form \(\mathrm{CH}_{3} \mathrm{COOH}\) from \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) ?
\({\text{(A) PCC}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(B)PDC}}\)
\({\text{(C) }}{{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{/}}{{\text{H}}^{\text{ + }}}\,\,\,\,\,\,\,\,\,{\text{(D)Micoderma}}\,\,{\text{aceti}}\)
323888 In the reaction, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{4}}}\xrightarrow{{{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}}}{\text{A}}\xrightarrow{{{{\text{H}}^{\text{ + }}}{\text{/}}{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{B}}\) A and B are
323890
Which of the following can't form \(\mathrm{CH}_{3} \mathrm{COOH}\) from \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) ?
\({\text{(A) PCC}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(B)PDC}}\)
\({\text{(C) }}{{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{/}}{{\text{H}}^{\text{ + }}}\,\,\,\,\,\,\,\,\,{\text{(D)Micoderma}}\,\,{\text{aceti}}\)
323888 In the reaction, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COON}}{{\text{H}}_{\text{4}}}\xrightarrow{{{{\text{P}}_{\text{2}}}{{\text{O}}_{\text{5}}}}}{\text{A}}\xrightarrow{{{{\text{H}}^{\text{ + }}}{\text{/}}{{\text{H}}_{\text{2}}}{\text{O}}}}{\text{B}}\) A and B are
323890
Which of the following can't form \(\mathrm{CH}_{3} \mathrm{COOH}\) from \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) ?
\({\text{(A) PCC}}\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,\,{\text{(B)PDC}}\)
\({\text{(C) }}{{\text{K}}_{\text{2}}}{\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{2}}}{\text{/}}{{\text{H}}^{\text{ + }}}\,\,\,\,\,\,\,\,\,{\text{(D)Micoderma}}\,\,{\text{aceti}}\)