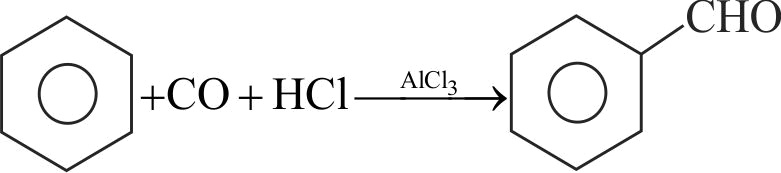
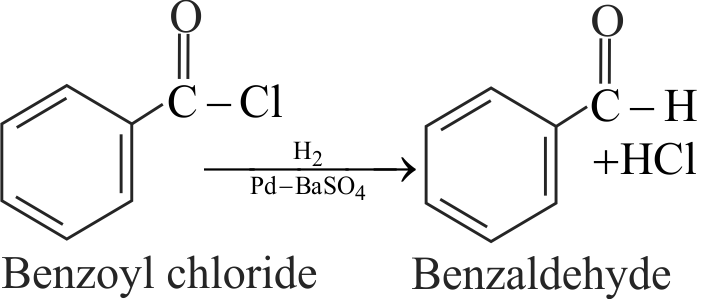
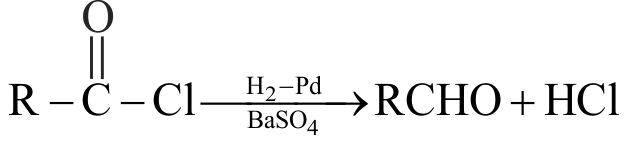323832 Compound 'A' is \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\xrightarrow[{\left( {\text{2}} \right)\,{{\text{H}}_{\text{2}}}{\text{O}}\,}]{{\left( {\text{1}} \right)\,\,{\text{DIBAL - H}}}}{\text{A}}{\text{.}}\)
323832 Compound 'A' is \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\xrightarrow[{\left( {\text{2}} \right)\,{{\text{H}}_{\text{2}}}{\text{O}}\,}]{{\left( {\text{1}} \right)\,\,{\text{DIBAL - H}}}}{\text{A}}{\text{.}}\)
323832 Compound 'A' is \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\xrightarrow[{\left( {\text{2}} \right)\,{{\text{H}}_{\text{2}}}{\text{O}}\,}]{{\left( {\text{1}} \right)\,\,{\text{DIBAL - H}}}}{\text{A}}{\text{.}}\)
323832 Compound 'A' is \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\xrightarrow[{\left( {\text{2}} \right)\,{{\text{H}}_{\text{2}}}{\text{O}}\,}]{{\left( {\text{1}} \right)\,\,{\text{DIBAL - H}}}}{\text{A}}{\text{.}}\)