323831
Assertion :
Formaldehyde cannot be prepared by Rosenmund's reduction.
Reason :
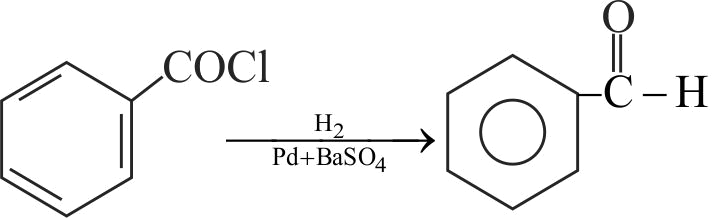
Acid chlorides can be reduced into aldehydes with hydrogen in boiling xylene using palladium or platinum as a catalyst supported on barium sulphate. This is known as
Rosenmund's reaction
323831
Assertion :
Formaldehyde cannot be prepared by Rosenmund's reduction.
Reason :
Acid chlorides can be reduced into aldehydes with hydrogen in boiling xylene using palladium or platinum as a catalyst supported on barium sulphate. This is known as
Rosenmund's reaction
323831
Assertion :
Formaldehyde cannot be prepared by Rosenmund's reduction.
Reason :
Acid chlorides can be reduced into aldehydes with hydrogen in boiling xylene using palladium or platinum as a catalyst supported on barium sulphate. This is known as
Rosenmund's reaction
323831
Assertion :
Formaldehyde cannot be prepared by Rosenmund's reduction.
Reason :
Acid chlorides can be reduced into aldehydes with hydrogen in boiling xylene using palladium or platinum as a catalyst supported on barium sulphate. This is known as
Rosenmund's reaction