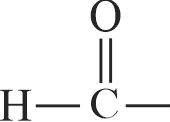323667
\({\text{C}}{{\text{H}}_{\text{3}}} - {\text{CHO + 2C}}{{\text{u}}^{{\text{2 + }}}}{\text{ + 5O}}{{\text{H}}^{{ - }}}\)
\(\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\xrightarrow{\Delta }\) Red brown ppt
The change that occurs in the reaction is
\({\mathrm{\mathrm{Cu}^{2+} \longrightarrow \mathrm{Cu}^{x+}}}\).
The value of \({\mathrm{x}}\) is ____ .
323667
\({\text{C}}{{\text{H}}_{\text{3}}} - {\text{CHO + 2C}}{{\text{u}}^{{\text{2 + }}}}{\text{ + 5O}}{{\text{H}}^{{ - }}}\)
\(\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\xrightarrow{\Delta }\) Red brown ppt
The change that occurs in the reaction is
\({\mathrm{\mathrm{Cu}^{2+} \longrightarrow \mathrm{Cu}^{x+}}}\).
The value of \({\mathrm{x}}\) is ____ .
323667
\({\text{C}}{{\text{H}}_{\text{3}}} - {\text{CHO + 2C}}{{\text{u}}^{{\text{2 + }}}}{\text{ + 5O}}{{\text{H}}^{{ - }}}\)
\(\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\xrightarrow{\Delta }\) Red brown ppt
The change that occurs in the reaction is
\({\mathrm{\mathrm{Cu}^{2+} \longrightarrow \mathrm{Cu}^{x+}}}\).
The value of \({\mathrm{x}}\) is ____ .
323667
\({\text{C}}{{\text{H}}_{\text{3}}} - {\text{CHO + 2C}}{{\text{u}}^{{\text{2 + }}}}{\text{ + 5O}}{{\text{H}}^{{ - }}}\)
\(\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\;\xrightarrow{\Delta }\) Red brown ppt
The change that occurs in the reaction is
\({\mathrm{\mathrm{Cu}^{2+} \longrightarrow \mathrm{Cu}^{x+}}}\).
The value of \({\mathrm{x}}\) is ____ .