323556
\[\begin{gathered}
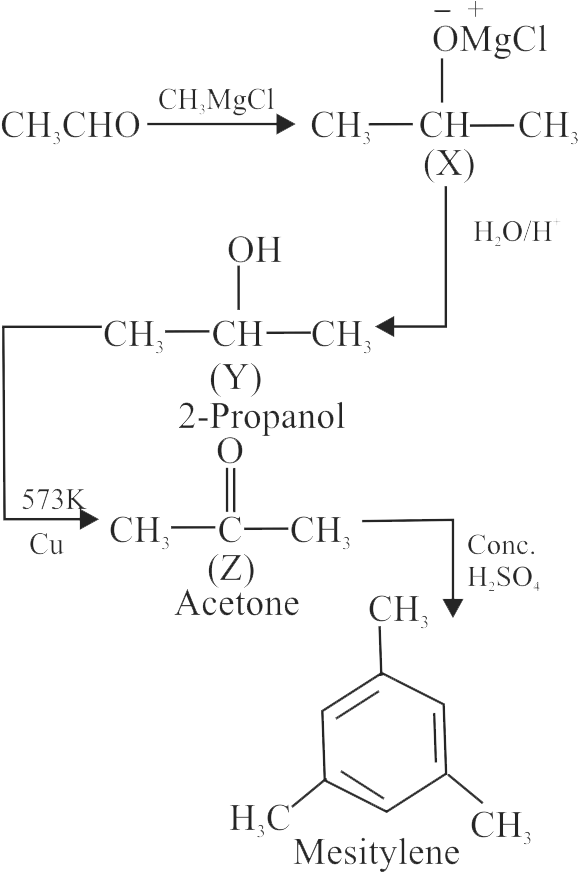
{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\xrightarrow{{{\text{C}}{{\text{H}}_{\text{3}}}{\text{MgCl}}}}{\text{X}}\xrightarrow{{{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}}}{\text{Y}} \hfill \\
\xrightarrow[{573\;{\text{K}}}]{{{\text{Cu}}}}{\text{Z}}\xrightarrow{{{\text{ Conc}}{\text{. }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{ Mesitylene }} \hfill \\
\end{gathered} \]
Identify the substance ' Y '.
323556
\[\begin{gathered}
{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\xrightarrow{{{\text{C}}{{\text{H}}_{\text{3}}}{\text{MgCl}}}}{\text{X}}\xrightarrow{{{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}}}{\text{Y}} \hfill \\
\xrightarrow[{573\;{\text{K}}}]{{{\text{Cu}}}}{\text{Z}}\xrightarrow{{{\text{ Conc}}{\text{. }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{ Mesitylene }} \hfill \\
\end{gathered} \]
Identify the substance ' Y '.
323556
\[\begin{gathered}
{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\xrightarrow{{{\text{C}}{{\text{H}}_{\text{3}}}{\text{MgCl}}}}{\text{X}}\xrightarrow{{{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}}}{\text{Y}} \hfill \\
\xrightarrow[{573\;{\text{K}}}]{{{\text{Cu}}}}{\text{Z}}\xrightarrow{{{\text{ Conc}}{\text{. }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{ Mesitylene }} \hfill \\
\end{gathered} \]
Identify the substance ' Y '.
323556
\[\begin{gathered}
{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\xrightarrow{{{\text{C}}{{\text{H}}_{\text{3}}}{\text{MgCl}}}}{\text{X}}\xrightarrow{{{{\text{H}}_{\text{3}}}{{\text{O}}^{\text{ + }}}}}{\text{Y}} \hfill \\
\xrightarrow[{573\;{\text{K}}}]{{{\text{Cu}}}}{\text{Z}}\xrightarrow{{{\text{ Conc}}{\text{. }}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{ Mesitylene }} \hfill \\
\end{gathered} \]
Identify the substance ' Y '.

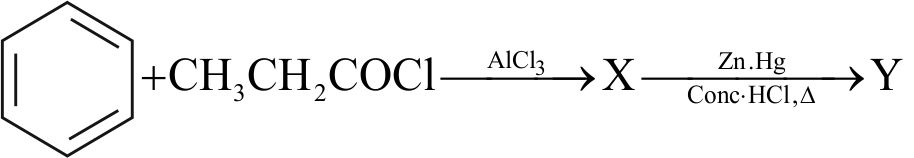
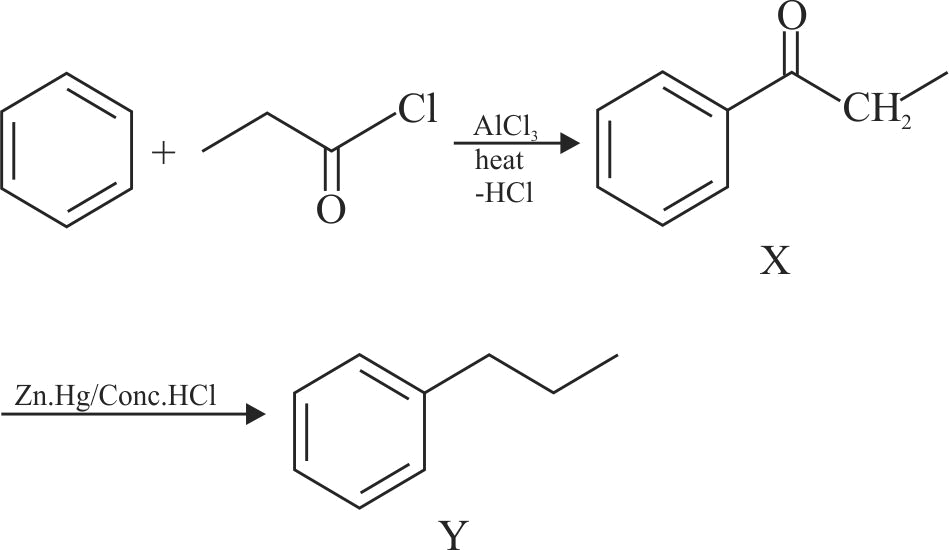
.png)