323284
Read the Statement - A and Statement - B carefully to mark the correct options given below
Statement A :
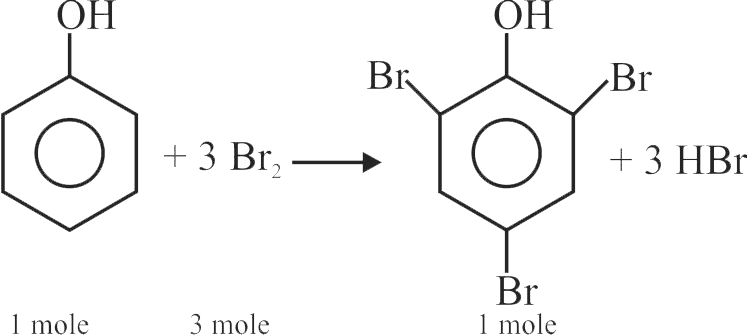
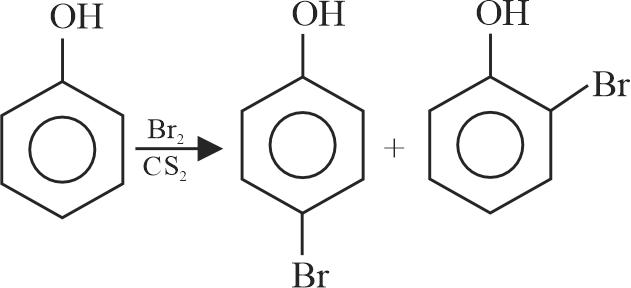
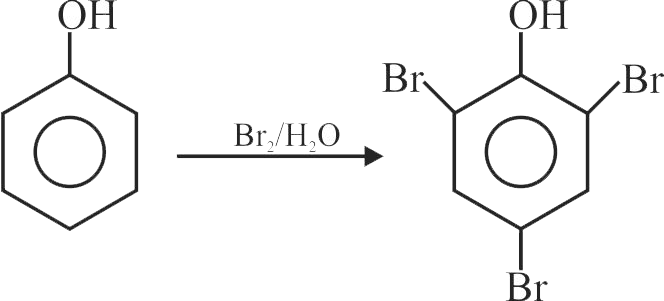
Bromination of phenol in solvent with low polarity such as \({\mathrm{\mathrm{CHCl}_{3}}}\) or \({\mathrm{\mathrm{CS}_{2}}}\) requires Lewis acid catalyst.
Statement B :
The Lewis acid catalyst polarises the bromine to generate \({\mathrm{\mathrm{Br}^{+}}}\).
323284
Read the Statement - A and Statement - B carefully to mark the correct options given below
Statement A :
Bromination of phenol in solvent with low polarity such as \({\mathrm{\mathrm{CHCl}_{3}}}\) or \({\mathrm{\mathrm{CS}_{2}}}\) requires Lewis acid catalyst.
Statement B :
The Lewis acid catalyst polarises the bromine to generate \({\mathrm{\mathrm{Br}^{+}}}\).
323284
Read the Statement - A and Statement - B carefully to mark the correct options given below
Statement A :
Bromination of phenol in solvent with low polarity such as \({\mathrm{\mathrm{CHCl}_{3}}}\) or \({\mathrm{\mathrm{CS}_{2}}}\) requires Lewis acid catalyst.
Statement B :
The Lewis acid catalyst polarises the bromine to generate \({\mathrm{\mathrm{Br}^{+}}}\).
323284
Read the Statement - A and Statement - B carefully to mark the correct options given below
Statement A :
Bromination of phenol in solvent with low polarity such as \({\mathrm{\mathrm{CHCl}_{3}}}\) or \({\mathrm{\mathrm{CS}_{2}}}\) requires Lewis acid catalyst.
Statement B :
The Lewis acid catalyst polarises the bromine to generate \({\mathrm{\mathrm{Br}^{+}}}\).