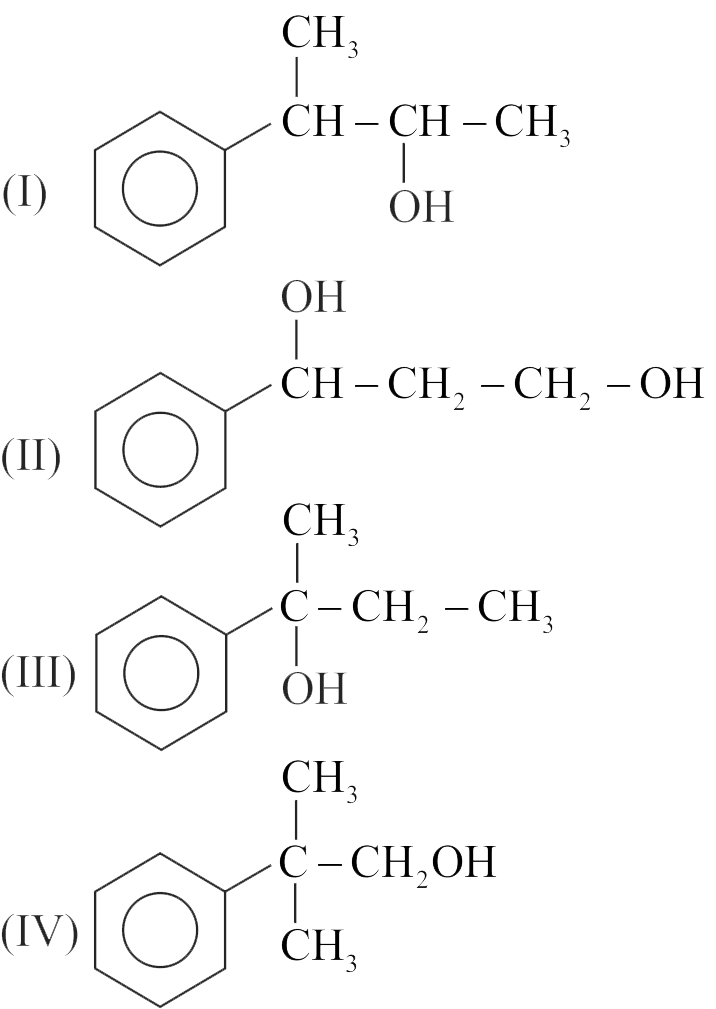
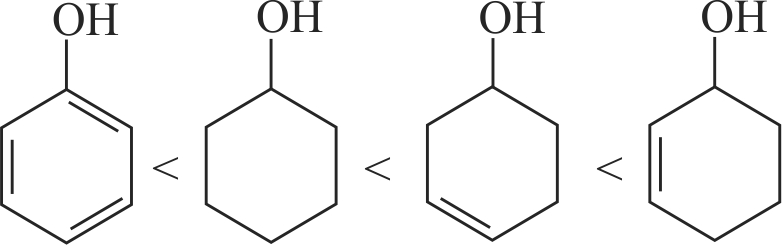
323100
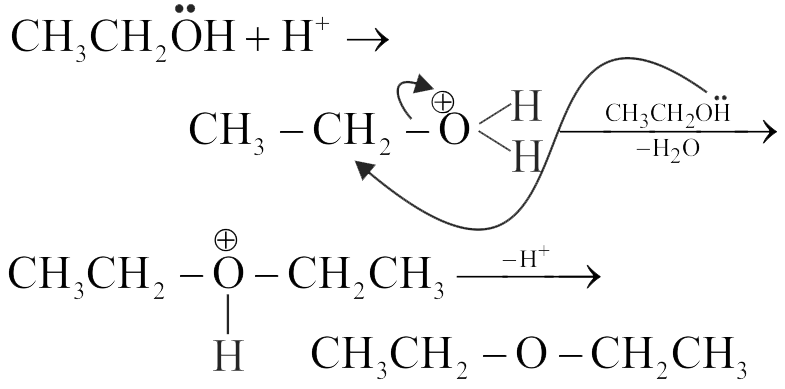
At 413 K , ethanol is dehydrated to produce ethoxyethane.
\(\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{OH} \xrightarrow[413 \mathrm{~K}]{\mathrm{H}_{2} \mathrm{SO}_{4}} \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OC}_{2} \mathrm{H}_{5}\)
Read the statements given below, and choose the option containing the correct statements.
I. Such a reaction is suitable for the preparation of ethers having primary alkyl groups only.
II. The mechanism involves the following sequence Protonation of alcohol \(\rightarrow\) Attack of another alcohol molecule on protonated alcohol \(\rightarrow\) Deprotonation.
III. At 443 K , the same reaction would produce ethene as the major product.
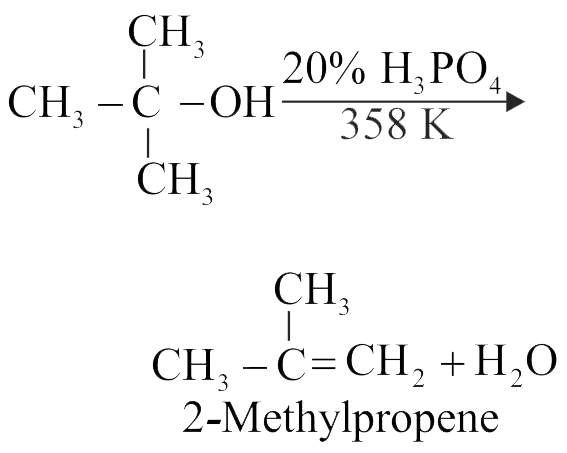
323101 When a tertiary alcohol ' A ' \({\mathrm{\left(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\right)}}\) reacts with \({\mathrm{20 \%\, \mathrm{H}_{3} \mathrm{PO}_{4}}}\) at 358 K , it gives a compound 'B' \({\mathrm{\left(\mathrm{C}_{4} \mathrm{H}_{8}\right)}}\) as a major product. The IUPAC name of the compound ' \({\mathrm{B}}\) ' is
323100
At 413 K , ethanol is dehydrated to produce ethoxyethane.
\(\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{OH} \xrightarrow[413 \mathrm{~K}]{\mathrm{H}_{2} \mathrm{SO}_{4}} \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OC}_{2} \mathrm{H}_{5}\)
Read the statements given below, and choose the option containing the correct statements.
I. Such a reaction is suitable for the preparation of ethers having primary alkyl groups only.
II. The mechanism involves the following sequence Protonation of alcohol \(\rightarrow\) Attack of another alcohol molecule on protonated alcohol \(\rightarrow\) Deprotonation.
III. At 443 K , the same reaction would produce ethene as the major product.
323101 When a tertiary alcohol ' A ' \({\mathrm{\left(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\right)}}\) reacts with \({\mathrm{20 \%\, \mathrm{H}_{3} \mathrm{PO}_{4}}}\) at 358 K , it gives a compound 'B' \({\mathrm{\left(\mathrm{C}_{4} \mathrm{H}_{8}\right)}}\) as a major product. The IUPAC name of the compound ' \({\mathrm{B}}\) ' is
323100
At 413 K , ethanol is dehydrated to produce ethoxyethane.
\(\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{OH} \xrightarrow[413 \mathrm{~K}]{\mathrm{H}_{2} \mathrm{SO}_{4}} \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OC}_{2} \mathrm{H}_{5}\)
Read the statements given below, and choose the option containing the correct statements.
I. Such a reaction is suitable for the preparation of ethers having primary alkyl groups only.
II. The mechanism involves the following sequence Protonation of alcohol \(\rightarrow\) Attack of another alcohol molecule on protonated alcohol \(\rightarrow\) Deprotonation.
III. At 443 K , the same reaction would produce ethene as the major product.
323101 When a tertiary alcohol ' A ' \({\mathrm{\left(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\right)}}\) reacts with \({\mathrm{20 \%\, \mathrm{H}_{3} \mathrm{PO}_{4}}}\) at 358 K , it gives a compound 'B' \({\mathrm{\left(\mathrm{C}_{4} \mathrm{H}_{8}\right)}}\) as a major product. The IUPAC name of the compound ' \({\mathrm{B}}\) ' is
323100
At 413 K , ethanol is dehydrated to produce ethoxyethane.
\(\mathrm{CH}_{3}-\mathrm{CH}_{2}-\mathrm{OH} \xrightarrow[413 \mathrm{~K}]{\mathrm{H}_{2} \mathrm{SO}_{4}} \mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OC}_{2} \mathrm{H}_{5}\)
Read the statements given below, and choose the option containing the correct statements.
I. Such a reaction is suitable for the preparation of ethers having primary alkyl groups only.
II. The mechanism involves the following sequence Protonation of alcohol \(\rightarrow\) Attack of another alcohol molecule on protonated alcohol \(\rightarrow\) Deprotonation.
III. At 443 K , the same reaction would produce ethene as the major product.
323101 When a tertiary alcohol ' A ' \({\mathrm{\left(\mathrm{C}_{4} \mathrm{H}_{10} \mathrm{O}\right)}}\) reacts with \({\mathrm{20 \%\, \mathrm{H}_{3} \mathrm{PO}_{4}}}\) at 358 K , it gives a compound 'B' \({\mathrm{\left(\mathrm{C}_{4} \mathrm{H}_{8}\right)}}\) as a major product. The IUPAC name of the compound ' \({\mathrm{B}}\) ' is