323049
Assertion :
\(\mathrm{CH}_{3} \mathrm{OCH}_{3}\) and \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) has comparable molecular weight but boiling point of \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) is more than dimethyl ether.
Reason :
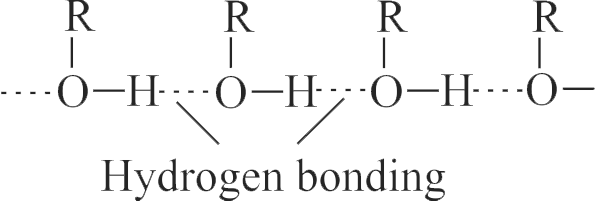
\(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) forms intermolecular \(\mathrm{H}\) -
bonding while \(\mathrm{CH}_{3} \mathrm{OCH}_{3}\) forms intramolecular \(\mathrm{H}\)-bonding.
323049
Assertion :
\(\mathrm{CH}_{3} \mathrm{OCH}_{3}\) and \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) has comparable molecular weight but boiling point of \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) is more than dimethyl ether.
Reason :
\(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) forms intermolecular \(\mathrm{H}\) -
bonding while \(\mathrm{CH}_{3} \mathrm{OCH}_{3}\) forms intramolecular \(\mathrm{H}\)-bonding.
323049
Assertion :
\(\mathrm{CH}_{3} \mathrm{OCH}_{3}\) and \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) has comparable molecular weight but boiling point of \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) is more than dimethyl ether.
Reason :
\(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) forms intermolecular \(\mathrm{H}\) -
bonding while \(\mathrm{CH}_{3} \mathrm{OCH}_{3}\) forms intramolecular \(\mathrm{H}\)-bonding.
323049
Assertion :
\(\mathrm{CH}_{3} \mathrm{OCH}_{3}\) and \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) has comparable molecular weight but boiling point of \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) is more than dimethyl ether.
Reason :
\(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) forms intermolecular \(\mathrm{H}\) -
bonding while \(\mathrm{CH}_{3} \mathrm{OCH}_{3}\) forms intramolecular \(\mathrm{H}\)-bonding.
323049
Assertion :
\(\mathrm{CH}_{3} \mathrm{OCH}_{3}\) and \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) has comparable molecular weight but boiling point of \(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) is more than dimethyl ether.
Reason :
\(\mathrm{C}_{2} \mathrm{H}_{5} \mathrm{OH}\) forms intermolecular \(\mathrm{H}\) -
bonding while \(\mathrm{CH}_{3} \mathrm{OCH}_{3}\) forms intramolecular \(\mathrm{H}\)-bonding.