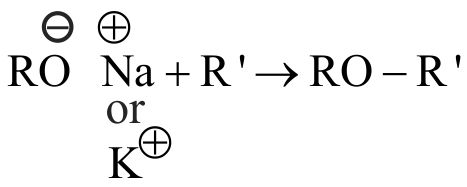322945
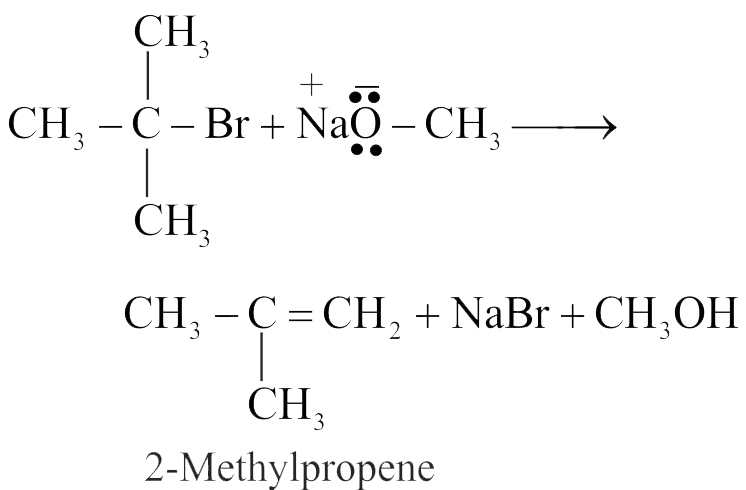
Which of the following on heating gives an ether as major product ?
\(\mathrm{P}: \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{Br}+\mathrm{CH}_{3} \mathrm{ONa}\)
Q : \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{ONa}+\mathrm{CH}_{3} \mathrm{Br}\)
\(\mathrm{R}:\left(\mathrm{CH}_{3}\right)_{3} \mathrm{C}-\mathrm{Cl}+\mathrm{CH}_{3} \mathrm{ONa}\)
\(\mathrm{S}: \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}=\mathrm{CHCl}+\mathrm{CH}_{3} \mathrm{ONa}\)
322946 The compound \({\text{A}}\) on treatment with \({\text{Na}}\) gives \({\text{B}}\), and with \({\text{PC}}{{\text{l}}_{\text{5}}}\) gives \({\text{C.}}{\mkern 1mu} \,{\text{B}}{\mkern 1mu} \,{\mkern 1mu} {\text{and}}{\mkern 1mu} \,{\text{C}}\) react together to give diethyl ether. \({\text{A,B}}\,\,{\text{and}}\,\,{\text{C}}\) are in the order
322945
Which of the following on heating gives an ether as major product ?
\(\mathrm{P}: \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{Br}+\mathrm{CH}_{3} \mathrm{ONa}\)
Q : \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{ONa}+\mathrm{CH}_{3} \mathrm{Br}\)
\(\mathrm{R}:\left(\mathrm{CH}_{3}\right)_{3} \mathrm{C}-\mathrm{Cl}+\mathrm{CH}_{3} \mathrm{ONa}\)
\(\mathrm{S}: \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}=\mathrm{CHCl}+\mathrm{CH}_{3} \mathrm{ONa}\)
322946 The compound \({\text{A}}\) on treatment with \({\text{Na}}\) gives \({\text{B}}\), and with \({\text{PC}}{{\text{l}}_{\text{5}}}\) gives \({\text{C.}}{\mkern 1mu} \,{\text{B}}{\mkern 1mu} \,{\mkern 1mu} {\text{and}}{\mkern 1mu} \,{\text{C}}\) react together to give diethyl ether. \({\text{A,B}}\,\,{\text{and}}\,\,{\text{C}}\) are in the order
322945
Which of the following on heating gives an ether as major product ?
\(\mathrm{P}: \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{Br}+\mathrm{CH}_{3} \mathrm{ONa}\)
Q : \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{ONa}+\mathrm{CH}_{3} \mathrm{Br}\)
\(\mathrm{R}:\left(\mathrm{CH}_{3}\right)_{3} \mathrm{C}-\mathrm{Cl}+\mathrm{CH}_{3} \mathrm{ONa}\)
\(\mathrm{S}: \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}=\mathrm{CHCl}+\mathrm{CH}_{3} \mathrm{ONa}\)
322946 The compound \({\text{A}}\) on treatment with \({\text{Na}}\) gives \({\text{B}}\), and with \({\text{PC}}{{\text{l}}_{\text{5}}}\) gives \({\text{C.}}{\mkern 1mu} \,{\text{B}}{\mkern 1mu} \,{\mkern 1mu} {\text{and}}{\mkern 1mu} \,{\text{C}}\) react together to give diethyl ether. \({\text{A,B}}\,\,{\text{and}}\,\,{\text{C}}\) are in the order
322945
Which of the following on heating gives an ether as major product ?
\(\mathrm{P}: \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{Br}+\mathrm{CH}_{3} \mathrm{ONa}\)
Q : \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{ONa}+\mathrm{CH}_{3} \mathrm{Br}\)
\(\mathrm{R}:\left(\mathrm{CH}_{3}\right)_{3} \mathrm{C}-\mathrm{Cl}+\mathrm{CH}_{3} \mathrm{ONa}\)
\(\mathrm{S}: \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}=\mathrm{CHCl}+\mathrm{CH}_{3} \mathrm{ONa}\)
322946 The compound \({\text{A}}\) on treatment with \({\text{Na}}\) gives \({\text{B}}\), and with \({\text{PC}}{{\text{l}}_{\text{5}}}\) gives \({\text{C.}}{\mkern 1mu} \,{\text{B}}{\mkern 1mu} \,{\mkern 1mu} {\text{and}}{\mkern 1mu} \,{\text{C}}\) react together to give diethyl ether. \({\text{A,B}}\,\,{\text{and}}\,\,{\text{C}}\) are in the order
322945
Which of the following on heating gives an ether as major product ?
\(\mathrm{P}: \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}_{2} \mathrm{Br}+\mathrm{CH}_{3} \mathrm{ONa}\)
Q : \(\mathrm{C}_{6} \mathrm{H}_{5} \mathrm{ONa}+\mathrm{CH}_{3} \mathrm{Br}\)
\(\mathrm{R}:\left(\mathrm{CH}_{3}\right)_{3} \mathrm{C}-\mathrm{Cl}+\mathrm{CH}_{3} \mathrm{ONa}\)
\(\mathrm{S}: \mathrm{C}_{6} \mathrm{H}_{5} \mathrm{CH}=\mathrm{CHCl}+\mathrm{CH}_{3} \mathrm{ONa}\)
322946 The compound \({\text{A}}\) on treatment with \({\text{Na}}\) gives \({\text{B}}\), and with \({\text{PC}}{{\text{l}}_{\text{5}}}\) gives \({\text{C.}}{\mkern 1mu} \,{\text{B}}{\mkern 1mu} \,{\mkern 1mu} {\text{and}}{\mkern 1mu} \,{\text{C}}\) react together to give diethyl ether. \({\text{A,B}}\,\,{\text{and}}\,\,{\text{C}}\) are in the order