322976
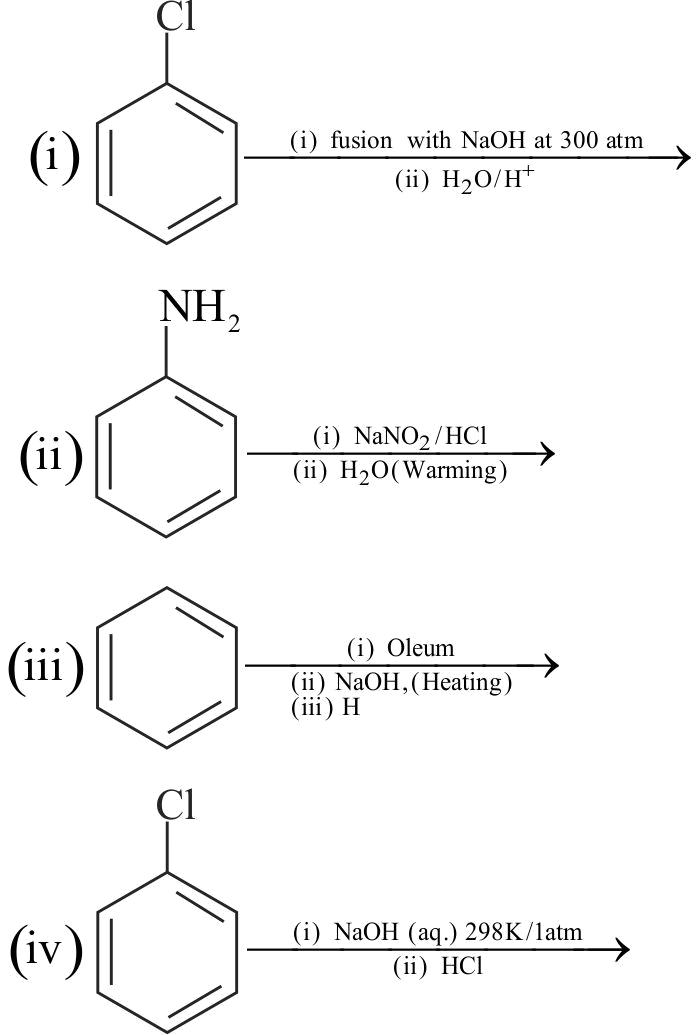
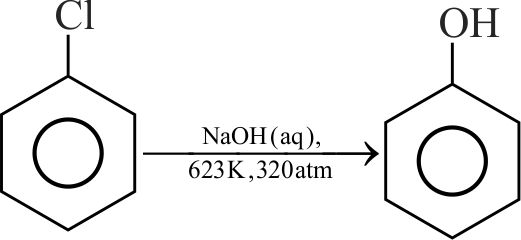
Which of the following reagents can be used for preparation of cumene?
(i) \(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{Cl}_{2}, \mathrm{hv} ; \mathrm{Mg} \cdot \mathrm{THF}\); acetone.
(ii) \(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{Cl}, \mathrm{AlCl}_{3}\).
(iii) \(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{CH}_{3} \mathrm{CHClCH}_{3}, \mathrm{AlCl}_{3}\).
(iv) \(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{Cl}, \mathrm{AlCl}_{3}\);
322978
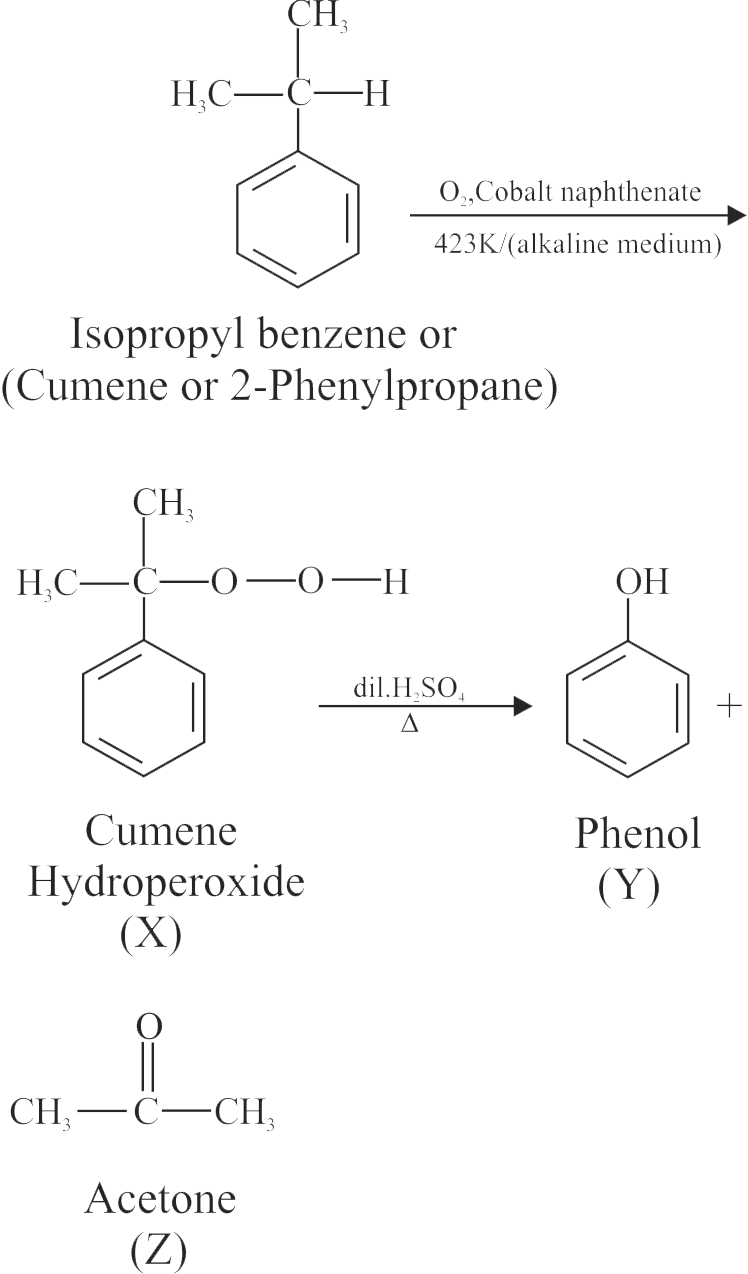
\({\text{Isopropyl}}\;{\text{benzene}}\xrightarrow[{{\text{423}}\;{\text{K/(alkaline}}\;{\text{medium)}}}]{{{{\text{O}}_{\text{2}}}{\text{,}}\;{\text{Cobalt}}\;{\text{naphthenate}}}}\)
\(\;\;\;\;\;\;\;\;\;\;\;\;{\text{X}}\xrightarrow[\Delta ]{{{\text{dil}}{\text{.}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{Y + Z}}\)
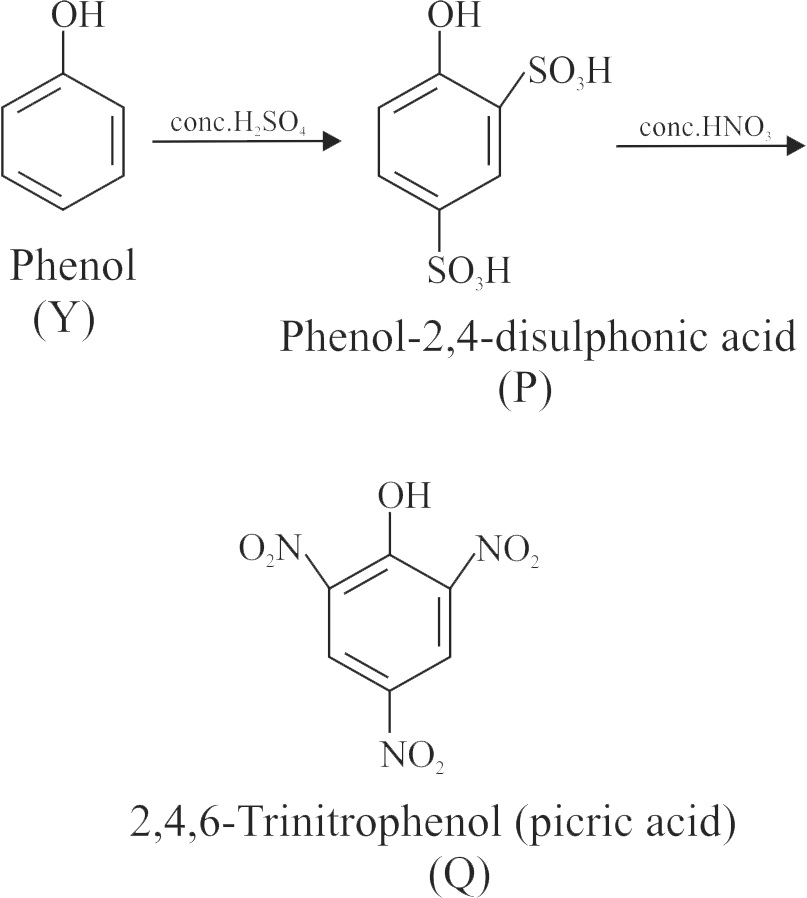
\({\text{Y}}\xrightarrow{{{\text{conc}}{\text{.}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{P}}\xrightarrow{{{\text{conc}}{\text{.HN}}{{\text{O}}_{\text{3}}}}}{\text{Q}}\)
How many electron withdrawing groups is/are present in the final product 'Q'?
322976
Which of the following reagents can be used for preparation of cumene?
(i) \(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{Cl}_{2}, \mathrm{hv} ; \mathrm{Mg} \cdot \mathrm{THF}\); acetone.
(ii) \(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{Cl}, \mathrm{AlCl}_{3}\).
(iii) \(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{CH}_{3} \mathrm{CHClCH}_{3}, \mathrm{AlCl}_{3}\).
(iv) \(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{Cl}, \mathrm{AlCl}_{3}\);
322978
\({\text{Isopropyl}}\;{\text{benzene}}\xrightarrow[{{\text{423}}\;{\text{K/(alkaline}}\;{\text{medium)}}}]{{{{\text{O}}_{\text{2}}}{\text{,}}\;{\text{Cobalt}}\;{\text{naphthenate}}}}\)
\(\;\;\;\;\;\;\;\;\;\;\;\;{\text{X}}\xrightarrow[\Delta ]{{{\text{dil}}{\text{.}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{Y + Z}}\)
\({\text{Y}}\xrightarrow{{{\text{conc}}{\text{.}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{P}}\xrightarrow{{{\text{conc}}{\text{.HN}}{{\text{O}}_{\text{3}}}}}{\text{Q}}\)
How many electron withdrawing groups is/are present in the final product 'Q'?
322976
Which of the following reagents can be used for preparation of cumene?
(i) \(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{Cl}_{2}, \mathrm{hv} ; \mathrm{Mg} \cdot \mathrm{THF}\); acetone.
(ii) \(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{Cl}, \mathrm{AlCl}_{3}\).
(iii) \(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{CH}_{3} \mathrm{CHClCH}_{3}, \mathrm{AlCl}_{3}\).
(iv) \(\mathrm{C}_{6} \mathrm{H}_{6}, \mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{Cl}, \mathrm{AlCl}_{3}\);
322978
\({\text{Isopropyl}}\;{\text{benzene}}\xrightarrow[{{\text{423}}\;{\text{K/(alkaline}}\;{\text{medium)}}}]{{{{\text{O}}_{\text{2}}}{\text{,}}\;{\text{Cobalt}}\;{\text{naphthenate}}}}\)
\(\;\;\;\;\;\;\;\;\;\;\;\;{\text{X}}\xrightarrow[\Delta ]{{{\text{dil}}{\text{.}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{Y + Z}}\)
\({\text{Y}}\xrightarrow{{{\text{conc}}{\text{.}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}}}{\text{P}}\xrightarrow{{{\text{conc}}{\text{.HN}}{{\text{O}}_{\text{3}}}}}{\text{Q}}\)
How many electron withdrawing groups is/are present in the final product 'Q'?