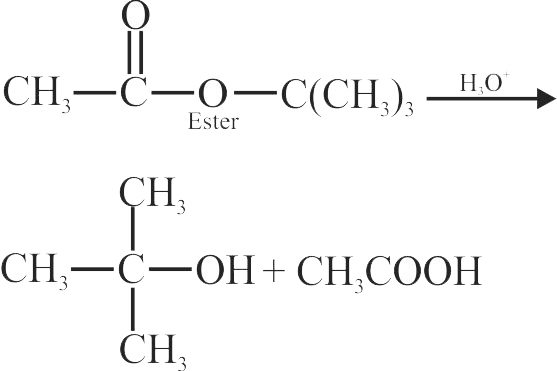
322927 Primary alcohol can easily be prepared from primary alkyl halide via \({{\text{S}}_{\text{N}}}^{\text{2}}\) reaction with aqueous \(\mathrm{NaOH}\). However, similar method does not work for the preparation of tertiary alcohol (tertiary butanol) from tertiary butyl bromide except
322927 Primary alcohol can easily be prepared from primary alkyl halide via \({{\text{S}}_{\text{N}}}^{\text{2}}\) reaction with aqueous \(\mathrm{NaOH}\). However, similar method does not work for the preparation of tertiary alcohol (tertiary butanol) from tertiary butyl bromide except
322927 Primary alcohol can easily be prepared from primary alkyl halide via \({{\text{S}}_{\text{N}}}^{\text{2}}\) reaction with aqueous \(\mathrm{NaOH}\). However, similar method does not work for the preparation of tertiary alcohol (tertiary butanol) from tertiary butyl bromide except
322927 Primary alcohol can easily be prepared from primary alkyl halide via \({{\text{S}}_{\text{N}}}^{\text{2}}\) reaction with aqueous \(\mathrm{NaOH}\). However, similar method does not work for the preparation of tertiary alcohol (tertiary butanol) from tertiary butyl bromide except
322927 Primary alcohol can easily be prepared from primary alkyl halide via \({{\text{S}}_{\text{N}}}^{\text{2}}\) reaction with aqueous \(\mathrm{NaOH}\). However, similar method does not work for the preparation of tertiary alcohol (tertiary butanol) from tertiary butyl bromide except