CHXII10:HALOALKANES AND HALOARENES
322474
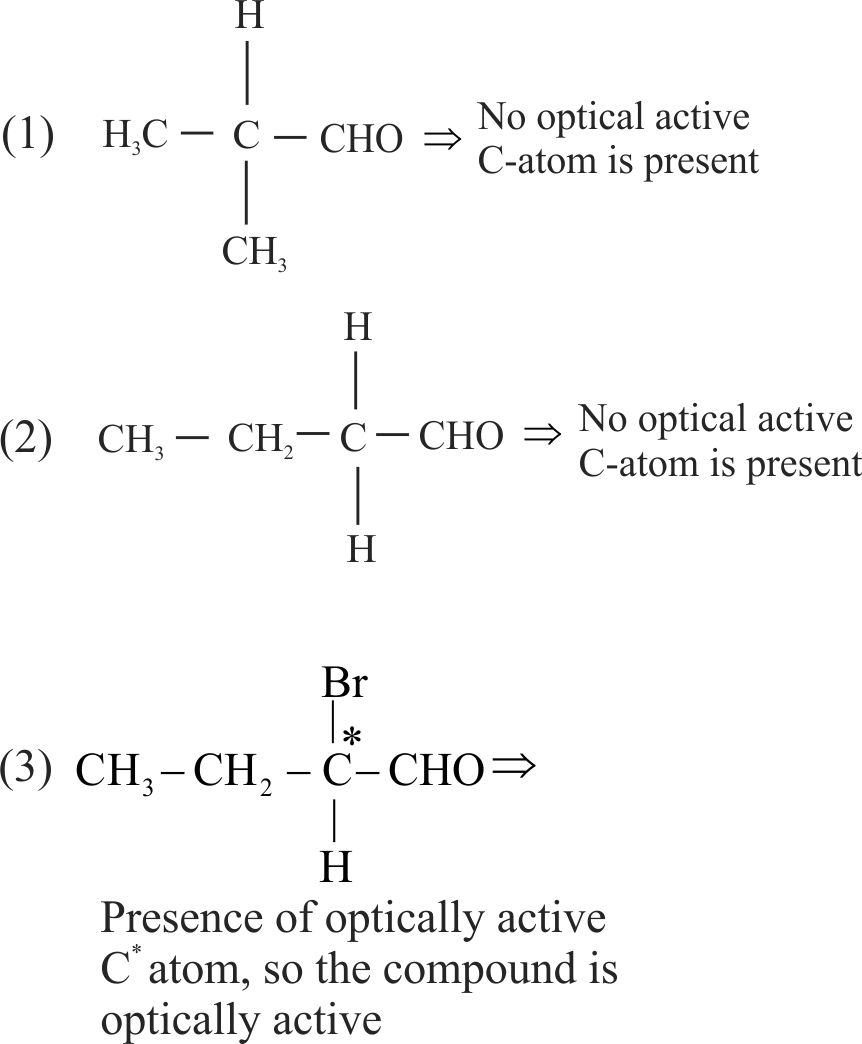
Which of the following compound is expected to be optically active?
1 \(\left(\mathrm{CH}_{3}\right)_{2} \mathrm{CHCHO}\)
2 \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}_{2} \mathrm{CHO}\)
3 \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CH}(\mathrm{Br}) \mathrm{CHO}\)
4 \(\mathrm{CH}_{3} \mathrm{CH}_{2} \mathrm{CBr}_{2} \mathrm{CHO}\)
Explanation:
An organic compound contains Chiral carbon (carbon attached to 4 different groups) can showoptical activity.