322328
Which of the following statements are correct?
(A) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same value of CFSE
(B) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same value of magnetic moment.
(C) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same hybridisation of nickel.
322329
Magnetic moment of complexes
(I) \(\left[\mathrm{Ni}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{1-}\)
(II) \(\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3} \mathrm{~F}_{3}\right]^{0}\)
(III) \(\left[\mathrm{Fe}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{1-}\)
(IV) \(\left[\mathrm{Cr}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{0}\)
are respectively (in B.M. units)
322330
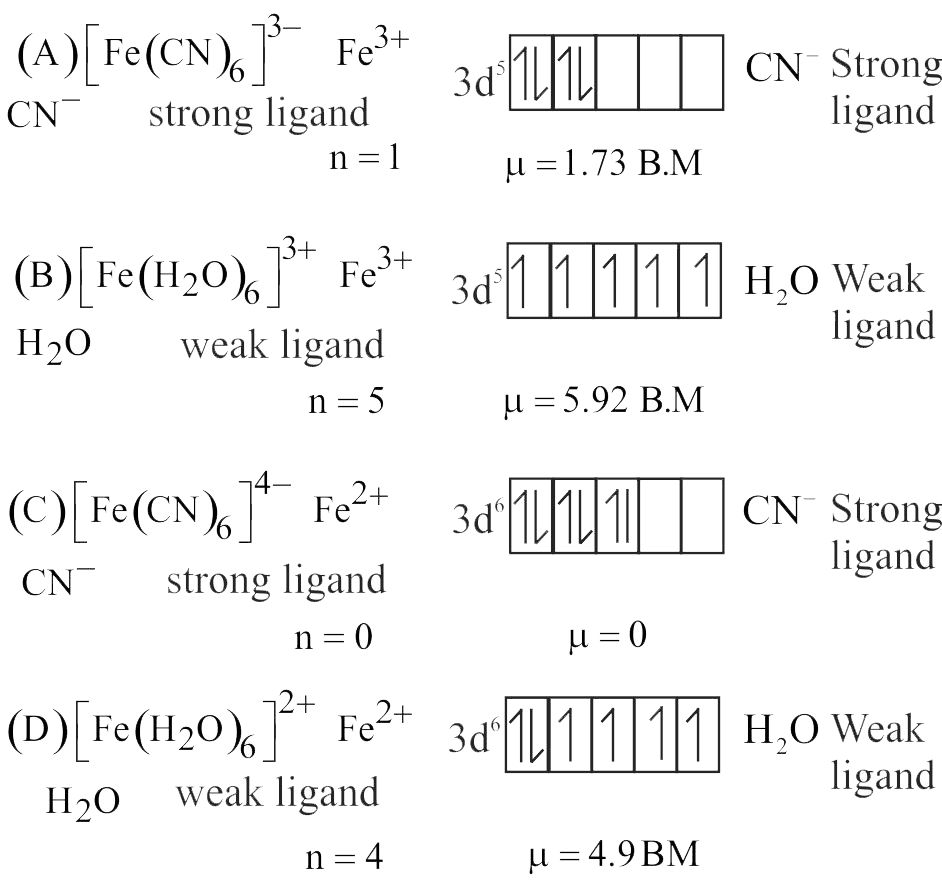
Statement A :
\(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) is paramagnetic while \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) is diamagnetic.
Statement B :
\(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) has +3 oxidation state while \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) has +2 oxidation state in terms of Fe.
322332
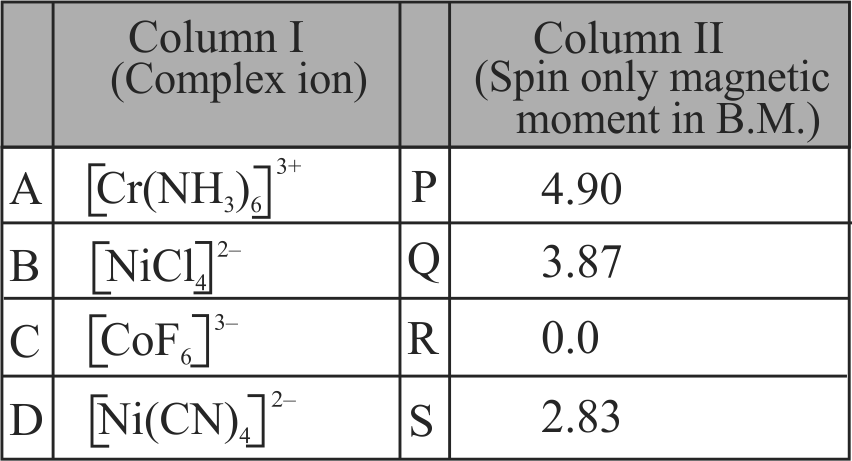
Match List - I with List - II.
Choose the correct answer from the options given below.
Column I
Column II
A
\({\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}\)
P
\(5.92\,\,{\text{BM}}\)
B
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+3}\)
Q
\({\text{0}}\,\,{\text{BM}}\)
C
\({\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{ - 4}}}}\)
R
\(4.90\,\,{\text{BM}}\)
D
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+2}\)
S
\(1.73\,\,{\text{BM}}\)
322328
Which of the following statements are correct?
(A) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same value of CFSE
(B) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same value of magnetic moment.
(C) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same hybridisation of nickel.
322329
Magnetic moment of complexes
(I) \(\left[\mathrm{Ni}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{1-}\)
(II) \(\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3} \mathrm{~F}_{3}\right]^{0}\)
(III) \(\left[\mathrm{Fe}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{1-}\)
(IV) \(\left[\mathrm{Cr}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{0}\)
are respectively (in B.M. units)
322330
Statement A :
\(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) is paramagnetic while \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) is diamagnetic.
Statement B :
\(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) has +3 oxidation state while \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) has +2 oxidation state in terms of Fe.
322332
Match List - I with List - II.
Choose the correct answer from the options given below.
Column I
Column II
A
\({\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}\)
P
\(5.92\,\,{\text{BM}}\)
B
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+3}\)
Q
\({\text{0}}\,\,{\text{BM}}\)
C
\({\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{ - 4}}}}\)
R
\(4.90\,\,{\text{BM}}\)
D
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+2}\)
S
\(1.73\,\,{\text{BM}}\)
322328
Which of the following statements are correct?
(A) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same value of CFSE
(B) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same value of magnetic moment.
(C) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same hybridisation of nickel.
322329
Magnetic moment of complexes
(I) \(\left[\mathrm{Ni}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{1-}\)
(II) \(\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3} \mathrm{~F}_{3}\right]^{0}\)
(III) \(\left[\mathrm{Fe}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{1-}\)
(IV) \(\left[\mathrm{Cr}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{0}\)
are respectively (in B.M. units)
322330
Statement A :
\(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) is paramagnetic while \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) is diamagnetic.
Statement B :
\(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) has +3 oxidation state while \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) has +2 oxidation state in terms of Fe.
322332
Match List - I with List - II.
Choose the correct answer from the options given below.
Column I
Column II
A
\({\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}\)
P
\(5.92\,\,{\text{BM}}\)
B
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+3}\)
Q
\({\text{0}}\,\,{\text{BM}}\)
C
\({\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{ - 4}}}}\)
R
\(4.90\,\,{\text{BM}}\)
D
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+2}\)
S
\(1.73\,\,{\text{BM}}\)
322328
Which of the following statements are correct?
(A) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same value of CFSE
(B) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same value of magnetic moment.
(C) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same hybridisation of nickel.
322329
Magnetic moment of complexes
(I) \(\left[\mathrm{Ni}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{1-}\)
(II) \(\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3} \mathrm{~F}_{3}\right]^{0}\)
(III) \(\left[\mathrm{Fe}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{1-}\)
(IV) \(\left[\mathrm{Cr}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{0}\)
are respectively (in B.M. units)
322330
Statement A :
\(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) is paramagnetic while \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) is diamagnetic.
Statement B :
\(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) has +3 oxidation state while \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) has +2 oxidation state in terms of Fe.
322332
Match List - I with List - II.
Choose the correct answer from the options given below.
Column I
Column II
A
\({\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}\)
P
\(5.92\,\,{\text{BM}}\)
B
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+3}\)
Q
\({\text{0}}\,\,{\text{BM}}\)
C
\({\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{ - 4}}}}\)
R
\(4.90\,\,{\text{BM}}\)
D
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+2}\)
S
\(1.73\,\,{\text{BM}}\)
322328
Which of the following statements are correct?
(A) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same value of CFSE
(B) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same value of magnetic moment.
(C) \(\left[\mathrm{Ni}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\) and \(\left[\mathrm{Ni}\left(\mathrm{NH}_{3}\right)_{6}\right]^{2+}\) have the same hybridisation of nickel.
322329
Magnetic moment of complexes
(I) \(\left[\mathrm{Ni}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{1-}\)
(II) \(\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3} \mathrm{~F}_{3}\right]^{0}\)
(III) \(\left[\mathrm{Fe}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{1-}\)
(IV) \(\left[\mathrm{Cr}(\mathrm{CN})_{3}\left(\mathrm{H}_{2} \mathrm{O}\right)_{3}\right]^{0}\)
are respectively (in B.M. units)
322330
Statement A :
\(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) is paramagnetic while \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) is diamagnetic.
Statement B :
\(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) has +3 oxidation state while \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{4-}\) has +2 oxidation state in terms of Fe.
322332
Match List - I with List - II.
Choose the correct answer from the options given below.
Column I
Column II
A
\({\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{3 - }}}}\)
P
\(5.92\,\,{\text{BM}}\)
B
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+3}\)
Q
\({\text{0}}\,\,{\text{BM}}\)
C
\({\left[ {{\text{Fe(CN}}{{\text{)}}_{\text{6}}}} \right]^{{\text{ - 4}}}}\)
R
\(4.90\,\,{\text{BM}}\)
D
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{+2}\)
S
\(1.73\,\,{\text{BM}}\)