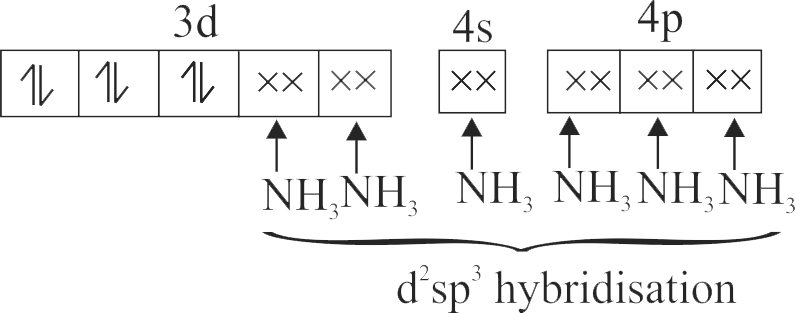
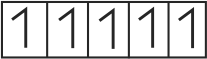
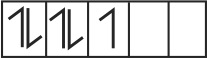
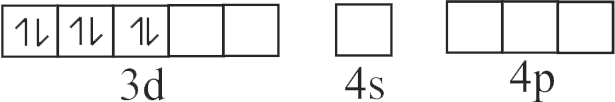
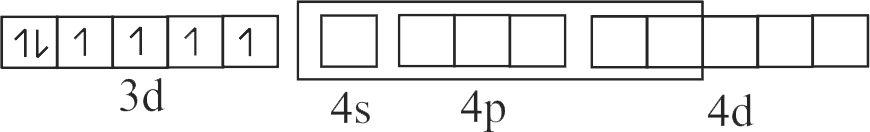
322303 \({\text{B}}{{\text{a}}^{{\text{2 + }}}}{\text{,C}}{{\text{N}}^{\text{ - }}}{\text{ & C}}{{\text{o}}^{{\text{2 + }}}}\) ions form an ionic complex. If this complex is \(75 \%\) ionised in aqueous solution with van't Hoff factor equal to 4 and \(\mu\) \((\) magnetic moment \()=1.73\) B.M. Then, the possible hybridisation of cobalt in the complex is
322306
Statement A :
Both \({\mathrm{\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}}}\) and
\({\mathrm{\left[\mathrm{CoF}_{6}\right]^{3-}}}\) complexes are octahedral but differ in their magnetic behaviour.
Statement B :
\({\mathrm{\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}}}\) is diamagnetic
whereas \({\mathrm{\left[\mathrm{CoF}_{6}\right]^{3-}}}\) is paramagnetic.
choose the correct answer from the options given below:
322303 \({\text{B}}{{\text{a}}^{{\text{2 + }}}}{\text{,C}}{{\text{N}}^{\text{ - }}}{\text{ & C}}{{\text{o}}^{{\text{2 + }}}}\) ions form an ionic complex. If this complex is \(75 \%\) ionised in aqueous solution with van't Hoff factor equal to 4 and \(\mu\) \((\) magnetic moment \()=1.73\) B.M. Then, the possible hybridisation of cobalt in the complex is
322306
Statement A :
Both \({\mathrm{\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}}}\) and
\({\mathrm{\left[\mathrm{CoF}_{6}\right]^{3-}}}\) complexes are octahedral but differ in their magnetic behaviour.
Statement B :
\({\mathrm{\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}}}\) is diamagnetic
whereas \({\mathrm{\left[\mathrm{CoF}_{6}\right]^{3-}}}\) is paramagnetic.
choose the correct answer from the options given below:
322303 \({\text{B}}{{\text{a}}^{{\text{2 + }}}}{\text{,C}}{{\text{N}}^{\text{ - }}}{\text{ & C}}{{\text{o}}^{{\text{2 + }}}}\) ions form an ionic complex. If this complex is \(75 \%\) ionised in aqueous solution with van't Hoff factor equal to 4 and \(\mu\) \((\) magnetic moment \()=1.73\) B.M. Then, the possible hybridisation of cobalt in the complex is
322306
Statement A :
Both \({\mathrm{\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}}}\) and
\({\mathrm{\left[\mathrm{CoF}_{6}\right]^{3-}}}\) complexes are octahedral but differ in their magnetic behaviour.
Statement B :
\({\mathrm{\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}}}\) is diamagnetic
whereas \({\mathrm{\left[\mathrm{CoF}_{6}\right]^{3-}}}\) is paramagnetic.
choose the correct answer from the options given below:
322303 \({\text{B}}{{\text{a}}^{{\text{2 + }}}}{\text{,C}}{{\text{N}}^{\text{ - }}}{\text{ & C}}{{\text{o}}^{{\text{2 + }}}}\) ions form an ionic complex. If this complex is \(75 \%\) ionised in aqueous solution with van't Hoff factor equal to 4 and \(\mu\) \((\) magnetic moment \()=1.73\) B.M. Then, the possible hybridisation of cobalt in the complex is
322306
Statement A :
Both \({\mathrm{\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}}}\) and
\({\mathrm{\left[\mathrm{CoF}_{6}\right]^{3-}}}\) complexes are octahedral but differ in their magnetic behaviour.
Statement B :
\({\mathrm{\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}}}\) is diamagnetic
whereas \({\mathrm{\left[\mathrm{CoF}_{6}\right]^{3-}}}\) is paramagnetic.
choose the correct answer from the options given below: