322298
Assertion :
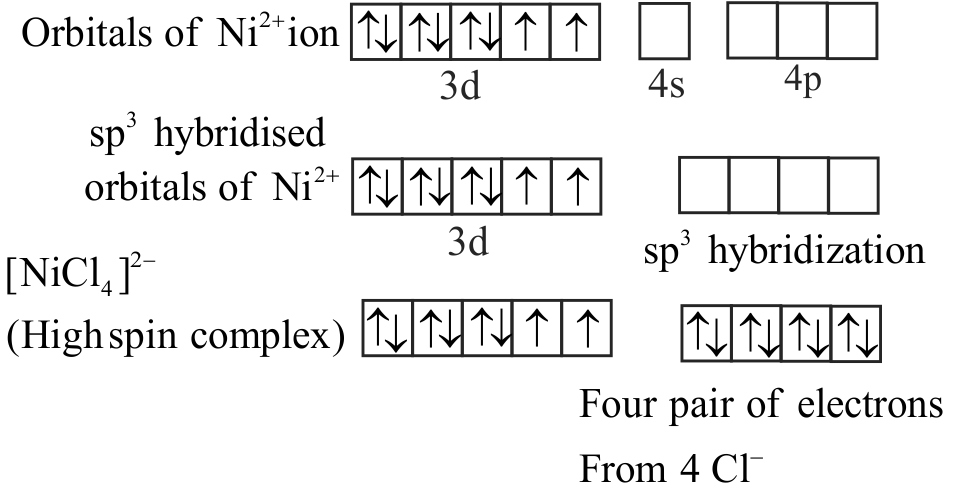
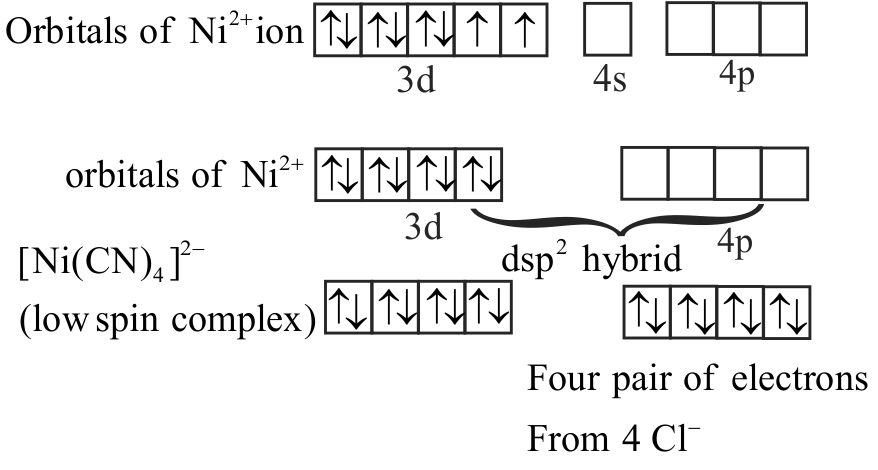
\(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}\) has square planar and
\(\left[\mathrm{NiCl}_{4}\right]^{2-}\) has tetrahedral shape.
Reason :
\(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}\) is diamagnetic while
\(\left[\mathrm{NiCl}_{4}\right]^{2-}\) is paramagnetic.
322299
Assertion :
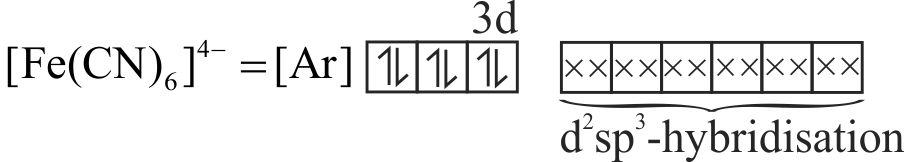
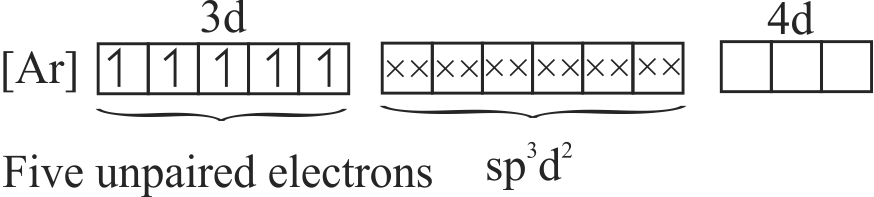
\(\mathrm{K}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]\) is diamagnetic and \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) is paramagnetic.
Reason :
Hybridisation of central metal in \(\mathrm{K}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]\) is \(\mathrm{sp}^{3} \mathrm{~d}^{2}\), while in\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) is \(\mathrm{d}^{2} \mathrm{sp}^{3}\).
322300
Assertion :
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}_{3} \mathrm{SO}_{4}\right.\) is
paramagnetic.
Reason :
The \(\mathrm{Fe}\) in \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}_{3} \mathrm{SO}_{4}\right.\) has three unpaired electrons.
322298
Assertion :
\(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}\) has square planar and
\(\left[\mathrm{NiCl}_{4}\right]^{2-}\) has tetrahedral shape.
Reason :
\(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}\) is diamagnetic while
\(\left[\mathrm{NiCl}_{4}\right]^{2-}\) is paramagnetic.
322299
Assertion :
\(\mathrm{K}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]\) is diamagnetic and \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) is paramagnetic.
Reason :
Hybridisation of central metal in \(\mathrm{K}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]\) is \(\mathrm{sp}^{3} \mathrm{~d}^{2}\), while in\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) is \(\mathrm{d}^{2} \mathrm{sp}^{3}\).
322300
Assertion :
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}_{3} \mathrm{SO}_{4}\right.\) is
paramagnetic.
Reason :
The \(\mathrm{Fe}\) in \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}_{3} \mathrm{SO}_{4}\right.\) has three unpaired electrons.
322298
Assertion :
\(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}\) has square planar and
\(\left[\mathrm{NiCl}_{4}\right]^{2-}\) has tetrahedral shape.
Reason :
\(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}\) is diamagnetic while
\(\left[\mathrm{NiCl}_{4}\right]^{2-}\) is paramagnetic.
322299
Assertion :
\(\mathrm{K}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]\) is diamagnetic and \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) is paramagnetic.
Reason :
Hybridisation of central metal in \(\mathrm{K}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]\) is \(\mathrm{sp}^{3} \mathrm{~d}^{2}\), while in\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) is \(\mathrm{d}^{2} \mathrm{sp}^{3}\).
322300
Assertion :
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}_{3} \mathrm{SO}_{4}\right.\) is
paramagnetic.
Reason :
The \(\mathrm{Fe}\) in \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}_{3} \mathrm{SO}_{4}\right.\) has three unpaired electrons.
322298
Assertion :
\(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}\) has square planar and
\(\left[\mathrm{NiCl}_{4}\right]^{2-}\) has tetrahedral shape.
Reason :
\(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}\) is diamagnetic while
\(\left[\mathrm{NiCl}_{4}\right]^{2-}\) is paramagnetic.
322299
Assertion :
\(\mathrm{K}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]\) is diamagnetic and \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) is paramagnetic.
Reason :
Hybridisation of central metal in \(\mathrm{K}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]\) is \(\mathrm{sp}^{3} \mathrm{~d}^{2}\), while in\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) is \(\mathrm{d}^{2} \mathrm{sp}^{3}\).
322300
Assertion :
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}_{3} \mathrm{SO}_{4}\right.\) is
paramagnetic.
Reason :
The \(\mathrm{Fe}\) in \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}_{3} \mathrm{SO}_{4}\right.\) has three unpaired electrons.
322298
Assertion :
\(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}\) has square planar and
\(\left[\mathrm{NiCl}_{4}\right]^{2-}\) has tetrahedral shape.
Reason :
\(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}\) is diamagnetic while
\(\left[\mathrm{NiCl}_{4}\right]^{2-}\) is paramagnetic.
322299
Assertion :
\(\mathrm{K}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]\) is diamagnetic and \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) is paramagnetic.
Reason :
Hybridisation of central metal in \(\mathrm{K}_{4}\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]\) is \(\mathrm{sp}^{3} \mathrm{~d}^{2}\), while in\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right] \mathrm{Cl}_{3}\) is \(\mathrm{d}^{2} \mathrm{sp}^{3}\).
322300
Assertion :
\(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}_{3} \mathrm{SO}_{4}\right.\) is
paramagnetic.
Reason :
The \(\mathrm{Fe}\) in \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{5} \mathrm{NO}_{3} \mathrm{SO}_{4}\right.\) has three unpaired electrons.