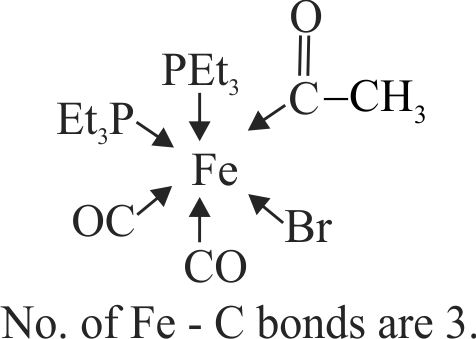
322249 In the complex acetyl bromido dicarbonyl bis (triethylphosphine) iron (II), the number of \(\mathrm{Fe}\) \(\mathrm{C}\) bonds is \(\left[\mathrm{Fe}\left(\mathrm{CH}_{3} \mathrm{CO}\right)(\mathrm{Br})(\mathrm{CO})_{2}\left\{\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{P}\right\}_{2}\right]\)
322249 In the complex acetyl bromido dicarbonyl bis (triethylphosphine) iron (II), the number of \(\mathrm{Fe}\) \(\mathrm{C}\) bonds is \(\left[\mathrm{Fe}\left(\mathrm{CH}_{3} \mathrm{CO}\right)(\mathrm{Br})(\mathrm{CO})_{2}\left\{\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{P}\right\}_{2}\right]\)
322249 In the complex acetyl bromido dicarbonyl bis (triethylphosphine) iron (II), the number of \(\mathrm{Fe}\) \(\mathrm{C}\) bonds is \(\left[\mathrm{Fe}\left(\mathrm{CH}_{3} \mathrm{CO}\right)(\mathrm{Br})(\mathrm{CO})_{2}\left\{\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{P}\right\}_{2}\right]\)
322249 In the complex acetyl bromido dicarbonyl bis (triethylphosphine) iron (II), the number of \(\mathrm{Fe}\) \(\mathrm{C}\) bonds is \(\left[\mathrm{Fe}\left(\mathrm{CH}_{3} \mathrm{CO}\right)(\mathrm{Br})(\mathrm{CO})_{2}\left\{\left(\mathrm{C}_{2} \mathrm{H}_{5}\right)_{3} \mathrm{P}\right\}_{2}\right]\)