322239
The correct order of the number of unpaired electrons in the given complexes is
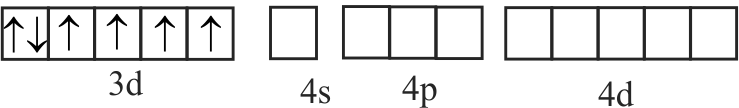
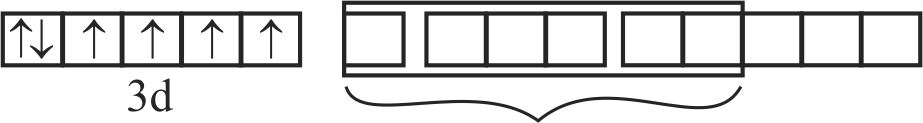
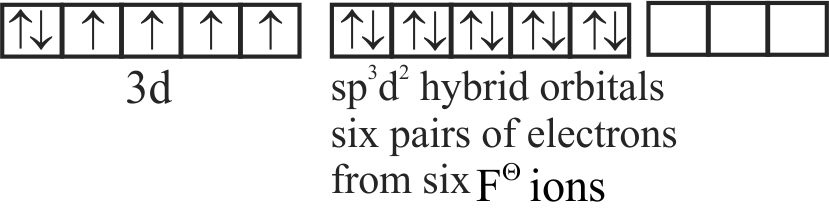
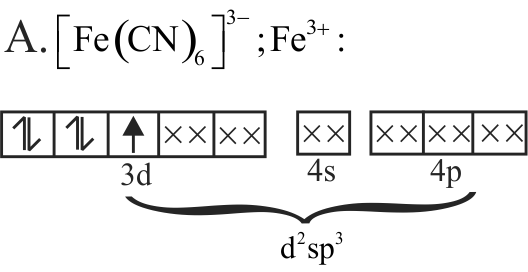
A. \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\)
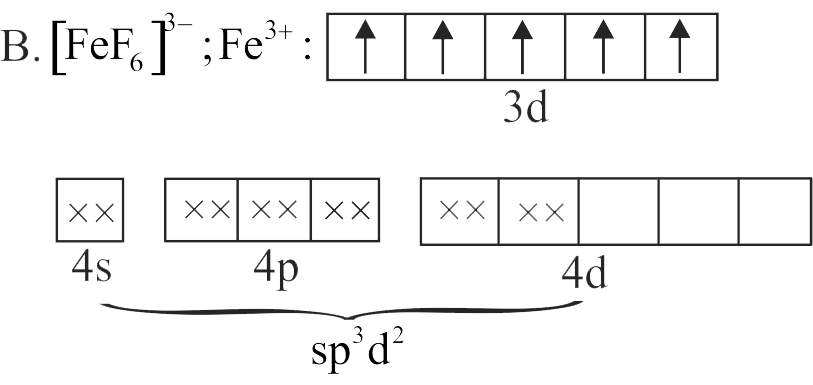
B. \(\left[\mathrm{FeF}_{6}\right]^{3-}\)
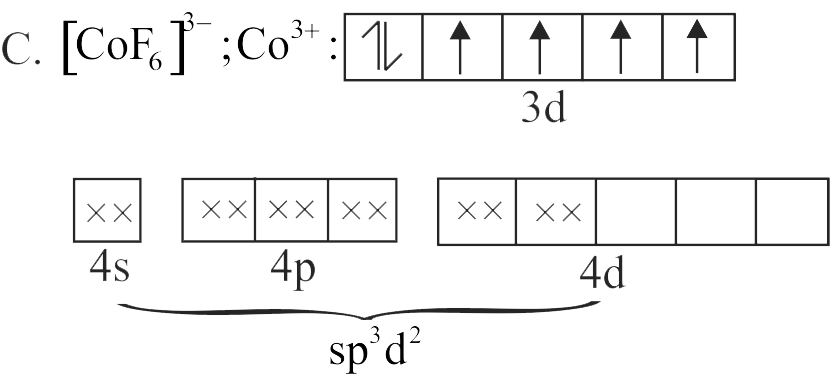
C. \({\left[ {{\rm{Co}}{{\rm{F}}_6}} \right]^{3 - }}\)
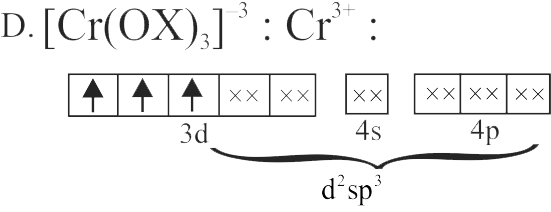
D. \(\left[\mathrm{Cr}(\text { oxalate })_{3}\right]^{3-}\)
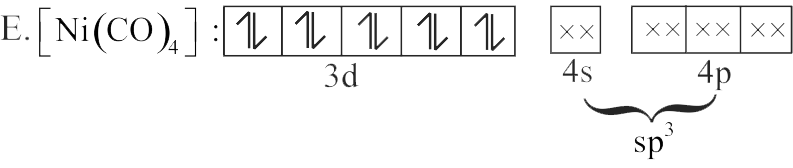
E. \(\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]\)
Choose the correct answer from the options given below:
322240
Among the following, the species having square planar geometry for central atom are
\({\text{I}}{\text{.Xe}}{{\text{F}}_{\text{4}}}\)
\({\text{II}}{\text{.S}}{{\text{F}}_{\text{4}}}\)
\({\text{III}}{\text{.}}{\left[ {{\text{NiC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}\)
\({\text{IV}}{\text{.}}{\left[ {{\text{PtC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}\)
322241
Assertion :
\(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) is coloured while \(\left[\mathrm{Cu}(\mathrm{CN})_{4}\right]^{3-}\) ion is colourless.
Reason :
\(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) has \(\mathrm{dsp}^{2}\) hybridisation.
322239
The correct order of the number of unpaired electrons in the given complexes is
A. \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\)
B. \(\left[\mathrm{FeF}_{6}\right]^{3-}\)
C. \({\left[ {{\rm{Co}}{{\rm{F}}_6}} \right]^{3 - }}\)
D. \(\left[\mathrm{Cr}(\text { oxalate })_{3}\right]^{3-}\)
E. \(\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]\)
Choose the correct answer from the options given below:
322240
Among the following, the species having square planar geometry for central atom are
\({\text{I}}{\text{.Xe}}{{\text{F}}_{\text{4}}}\)
\({\text{II}}{\text{.S}}{{\text{F}}_{\text{4}}}\)
\({\text{III}}{\text{.}}{\left[ {{\text{NiC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}\)
\({\text{IV}}{\text{.}}{\left[ {{\text{PtC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}\)
322241
Assertion :
\(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) is coloured while \(\left[\mathrm{Cu}(\mathrm{CN})_{4}\right]^{3-}\) ion is colourless.
Reason :
\(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) has \(\mathrm{dsp}^{2}\) hybridisation.
322239
The correct order of the number of unpaired electrons in the given complexes is
A. \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\)
B. \(\left[\mathrm{FeF}_{6}\right]^{3-}\)
C. \({\left[ {{\rm{Co}}{{\rm{F}}_6}} \right]^{3 - }}\)
D. \(\left[\mathrm{Cr}(\text { oxalate })_{3}\right]^{3-}\)
E. \(\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]\)
Choose the correct answer from the options given below:
322240
Among the following, the species having square planar geometry for central atom are
\({\text{I}}{\text{.Xe}}{{\text{F}}_{\text{4}}}\)
\({\text{II}}{\text{.S}}{{\text{F}}_{\text{4}}}\)
\({\text{III}}{\text{.}}{\left[ {{\text{NiC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}\)
\({\text{IV}}{\text{.}}{\left[ {{\text{PtC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}\)
322241
Assertion :
\(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) is coloured while \(\left[\mathrm{Cu}(\mathrm{CN})_{4}\right]^{3-}\) ion is colourless.
Reason :
\(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) has \(\mathrm{dsp}^{2}\) hybridisation.
322239
The correct order of the number of unpaired electrons in the given complexes is
A. \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\)
B. \(\left[\mathrm{FeF}_{6}\right]^{3-}\)
C. \({\left[ {{\rm{Co}}{{\rm{F}}_6}} \right]^{3 - }}\)
D. \(\left[\mathrm{Cr}(\text { oxalate })_{3}\right]^{3-}\)
E. \(\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]\)
Choose the correct answer from the options given below:
322240
Among the following, the species having square planar geometry for central atom are
\({\text{I}}{\text{.Xe}}{{\text{F}}_{\text{4}}}\)
\({\text{II}}{\text{.S}}{{\text{F}}_{\text{4}}}\)
\({\text{III}}{\text{.}}{\left[ {{\text{NiC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}\)
\({\text{IV}}{\text{.}}{\left[ {{\text{PtC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}\)
322241
Assertion :
\(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) is coloured while \(\left[\mathrm{Cu}(\mathrm{CN})_{4}\right]^{3-}\) ion is colourless.
Reason :
\(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) has \(\mathrm{dsp}^{2}\) hybridisation.
322239
The correct order of the number of unpaired electrons in the given complexes is
A. \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\)
B. \(\left[\mathrm{FeF}_{6}\right]^{3-}\)
C. \({\left[ {{\rm{Co}}{{\rm{F}}_6}} \right]^{3 - }}\)
D. \(\left[\mathrm{Cr}(\text { oxalate })_{3}\right]^{3-}\)
E. \(\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]\)
Choose the correct answer from the options given below:
322240
Among the following, the species having square planar geometry for central atom are
\({\text{I}}{\text{.Xe}}{{\text{F}}_{\text{4}}}\)
\({\text{II}}{\text{.S}}{{\text{F}}_{\text{4}}}\)
\({\text{III}}{\text{.}}{\left[ {{\text{NiC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}\)
\({\text{IV}}{\text{.}}{\left[ {{\text{PtC}}{{\text{l}}_{\text{4}}}} \right]^{{\text{2 - }}}}\)
322241
Assertion :
\(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) is coloured while \(\left[\mathrm{Cu}(\mathrm{CN})_{4}\right]^{3-}\) ion is colourless.
Reason :
\(\left[\mathrm{Cu}\left(\mathrm{NH}_{3}\right)_{4}\right]^{2+}\) has \(\mathrm{dsp}^{2}\) hybridisation.