322237
Which of the following complexes are not correctly matched with the hybridisation of their central metal ion?
(i) \(\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]=\mathrm{sp}^{3}\)
(ii) \(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}=\mathrm{sp}^{3}\)
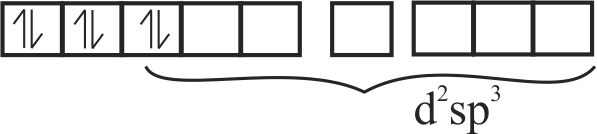
(iii) \(\left[\mathrm{CoF}_{6}\right]^{3-}=\mathrm{d}^{2} \mathrm{sp}^{3}\)
(iv) \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}=\mathrm{sp}^{3} \mathrm{~d}^{2}\)
Select the correct answer using the codes given below:
322237
Which of the following complexes are not correctly matched with the hybridisation of their central metal ion?
(i) \(\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]=\mathrm{sp}^{3}\)
(ii) \(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}=\mathrm{sp}^{3}\)
(iii) \(\left[\mathrm{CoF}_{6}\right]^{3-}=\mathrm{d}^{2} \mathrm{sp}^{3}\)
(iv) \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}=\mathrm{sp}^{3} \mathrm{~d}^{2}\)
Select the correct answer using the codes given below:
322237
Which of the following complexes are not correctly matched with the hybridisation of their central metal ion?
(i) \(\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]=\mathrm{sp}^{3}\)
(ii) \(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}=\mathrm{sp}^{3}\)
(iii) \(\left[\mathrm{CoF}_{6}\right]^{3-}=\mathrm{d}^{2} \mathrm{sp}^{3}\)
(iv) \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}=\mathrm{sp}^{3} \mathrm{~d}^{2}\)
Select the correct answer using the codes given below:
322237
Which of the following complexes are not correctly matched with the hybridisation of their central metal ion?
(i) \(\left[\mathrm{Ni}(\mathrm{CO})_{4}\right]=\mathrm{sp}^{3}\)
(ii) \(\left[\mathrm{Ni}(\mathrm{CN})_{4}\right]^{2-}=\mathrm{sp}^{3}\)
(iii) \(\left[\mathrm{CoF}_{6}\right]^{3-}=\mathrm{d}^{2} \mathrm{sp}^{3}\)
(iv) \(\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}=\mathrm{sp}^{3} \mathrm{~d}^{2}\)
Select the correct answer using the codes given below: