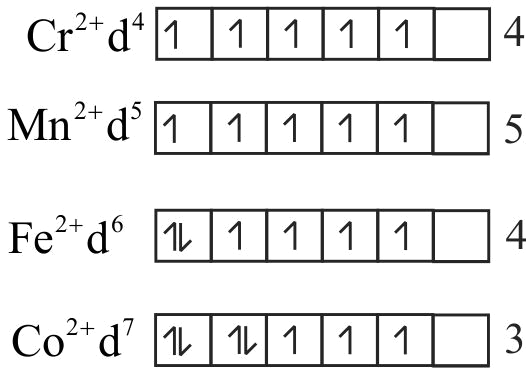
322341 The d-electron configuration of \(\mathrm{Cr}^{2+}, \mathrm{Mn}^{2+}, \mathrm{Fe}^{2+}\) and \(\mathrm{Co}^{2+}\) are \({{\text{d}}^{\text{4}}}{\text{,}}{{\text{d}}^{\text{5}}}{\text{,}}{{\text{d}}^{\text{6}}}{\text{and}}\,{{\text{d}}^{\text{7}}}{\text{,}}\) respectively. Which one of the following will exhibit minimum paramagnetic behaviour?(At, nos. \(\mathrm{Cr}=24, \mathrm{Mn}=25, \mathrm{Fe}=26, \mathrm{Co}=27\) )
322341 The d-electron configuration of \(\mathrm{Cr}^{2+}, \mathrm{Mn}^{2+}, \mathrm{Fe}^{2+}\) and \(\mathrm{Co}^{2+}\) are \({{\text{d}}^{\text{4}}}{\text{,}}{{\text{d}}^{\text{5}}}{\text{,}}{{\text{d}}^{\text{6}}}{\text{and}}\,{{\text{d}}^{\text{7}}}{\text{,}}\) respectively. Which one of the following will exhibit minimum paramagnetic behaviour?(At, nos. \(\mathrm{Cr}=24, \mathrm{Mn}=25, \mathrm{Fe}=26, \mathrm{Co}=27\) )
322341 The d-electron configuration of \(\mathrm{Cr}^{2+}, \mathrm{Mn}^{2+}, \mathrm{Fe}^{2+}\) and \(\mathrm{Co}^{2+}\) are \({{\text{d}}^{\text{4}}}{\text{,}}{{\text{d}}^{\text{5}}}{\text{,}}{{\text{d}}^{\text{6}}}{\text{and}}\,{{\text{d}}^{\text{7}}}{\text{,}}\) respectively. Which one of the following will exhibit minimum paramagnetic behaviour?(At, nos. \(\mathrm{Cr}=24, \mathrm{Mn}=25, \mathrm{Fe}=26, \mathrm{Co}=27\) )
322341 The d-electron configuration of \(\mathrm{Cr}^{2+}, \mathrm{Mn}^{2+}, \mathrm{Fe}^{2+}\) and \(\mathrm{Co}^{2+}\) are \({{\text{d}}^{\text{4}}}{\text{,}}{{\text{d}}^{\text{5}}}{\text{,}}{{\text{d}}^{\text{6}}}{\text{and}}\,{{\text{d}}^{\text{7}}}{\text{,}}\) respectively. Which one of the following will exhibit minimum paramagnetic behaviour?(At, nos. \(\mathrm{Cr}=24, \mathrm{Mn}=25, \mathrm{Fe}=26, \mathrm{Co}=27\) )