322339
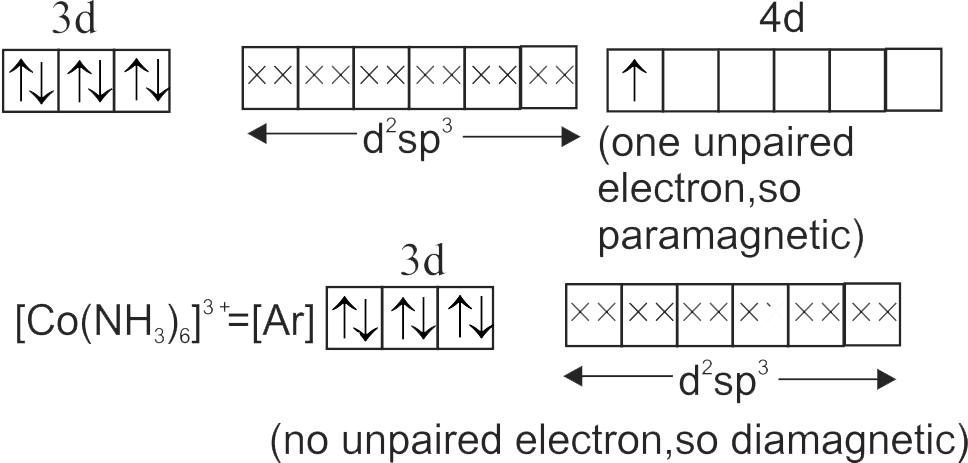
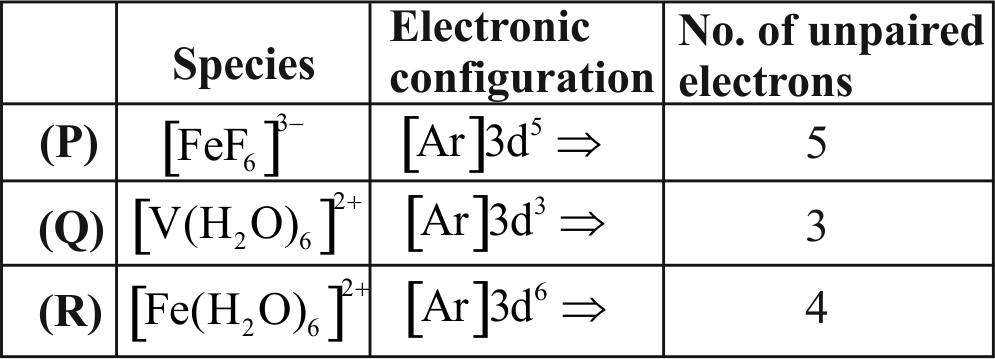
Consider the following complex ions
\(\mathrm{P}=\left[\mathrm{FeF}_{6}\right]^{3-} \mathrm{Q}=\left[\mathrm{V}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+} \mathrm{R}=\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\)
The correct order of the complex ions, according to their spin only magnetic moment values (in B.M.) is :
322340
If \(\mathrm{Ni}^{2+}\) is replaced by \(\mathrm{Pt}^{2+}\) in the complex \(\left[\mathrm{NiCl}_{2} \mathrm{Br}_{2}\right]^{2-}\), which of the following properties are expected to get changed?
A. Geometry
B. Geometrical isomerism
C. Optical isomerism
D. Magnetic properties
322339
Consider the following complex ions
\(\mathrm{P}=\left[\mathrm{FeF}_{6}\right]^{3-} \mathrm{Q}=\left[\mathrm{V}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+} \mathrm{R}=\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\)
The correct order of the complex ions, according to their spin only magnetic moment values (in B.M.) is :
322340
If \(\mathrm{Ni}^{2+}\) is replaced by \(\mathrm{Pt}^{2+}\) in the complex \(\left[\mathrm{NiCl}_{2} \mathrm{Br}_{2}\right]^{2-}\), which of the following properties are expected to get changed?
A. Geometry
B. Geometrical isomerism
C. Optical isomerism
D. Magnetic properties
322339
Consider the following complex ions
\(\mathrm{P}=\left[\mathrm{FeF}_{6}\right]^{3-} \mathrm{Q}=\left[\mathrm{V}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+} \mathrm{R}=\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\)
The correct order of the complex ions, according to their spin only magnetic moment values (in B.M.) is :
322340
If \(\mathrm{Ni}^{2+}\) is replaced by \(\mathrm{Pt}^{2+}\) in the complex \(\left[\mathrm{NiCl}_{2} \mathrm{Br}_{2}\right]^{2-}\), which of the following properties are expected to get changed?
A. Geometry
B. Geometrical isomerism
C. Optical isomerism
D. Magnetic properties
322339
Consider the following complex ions
\(\mathrm{P}=\left[\mathrm{FeF}_{6}\right]^{3-} \mathrm{Q}=\left[\mathrm{V}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+} \mathrm{R}=\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+}\)
The correct order of the complex ions, according to their spin only magnetic moment values (in B.M.) is :
322340
If \(\mathrm{Ni}^{2+}\) is replaced by \(\mathrm{Pt}^{2+}\) in the complex \(\left[\mathrm{NiCl}_{2} \mathrm{Br}_{2}\right]^{2-}\), which of the following properties are expected to get changed?
A. Geometry
B. Geometrical isomerism
C. Optical isomerism
D. Magnetic properties