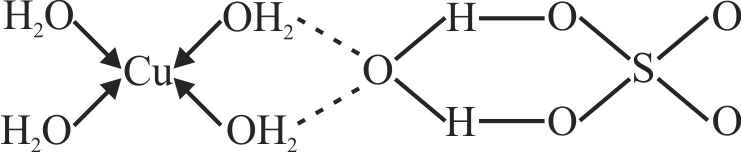
322219 \(\mathrm{FeSO}_{4}\) solution mixed with \(\left(\mathrm{NH}_{4}\right)_{2} \mathrm{SO}_{4}\) solution in 1:1 molar ratio gives the test of \(\mathrm{Fe}^{2+}\) ion but \(\mathrm{CuSO}_{4}\) solution mixed with aqueous ammonia in 1:4 molar ratio does not give the test of \(\mathrm{Cu}^{2+}\) ion. Explain why?
322219 \(\mathrm{FeSO}_{4}\) solution mixed with \(\left(\mathrm{NH}_{4}\right)_{2} \mathrm{SO}_{4}\) solution in 1:1 molar ratio gives the test of \(\mathrm{Fe}^{2+}\) ion but \(\mathrm{CuSO}_{4}\) solution mixed with aqueous ammonia in 1:4 molar ratio does not give the test of \(\mathrm{Cu}^{2+}\) ion. Explain why?
322219 \(\mathrm{FeSO}_{4}\) solution mixed with \(\left(\mathrm{NH}_{4}\right)_{2} \mathrm{SO}_{4}\) solution in 1:1 molar ratio gives the test of \(\mathrm{Fe}^{2+}\) ion but \(\mathrm{CuSO}_{4}\) solution mixed with aqueous ammonia in 1:4 molar ratio does not give the test of \(\mathrm{Cu}^{2+}\) ion. Explain why?
322219 \(\mathrm{FeSO}_{4}\) solution mixed with \(\left(\mathrm{NH}_{4}\right)_{2} \mathrm{SO}_{4}\) solution in 1:1 molar ratio gives the test of \(\mathrm{Fe}^{2+}\) ion but \(\mathrm{CuSO}_{4}\) solution mixed with aqueous ammonia in 1:4 molar ratio does not give the test of \(\mathrm{Cu}^{2+}\) ion. Explain why?