322091 In the complexes \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+},\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) \(\left[\mathrm{Fe}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{3}\right]^{3-}\) and \(\left[\mathrm{FeCl}_{6}\right]^{3-}\), more stability is shown by
322094
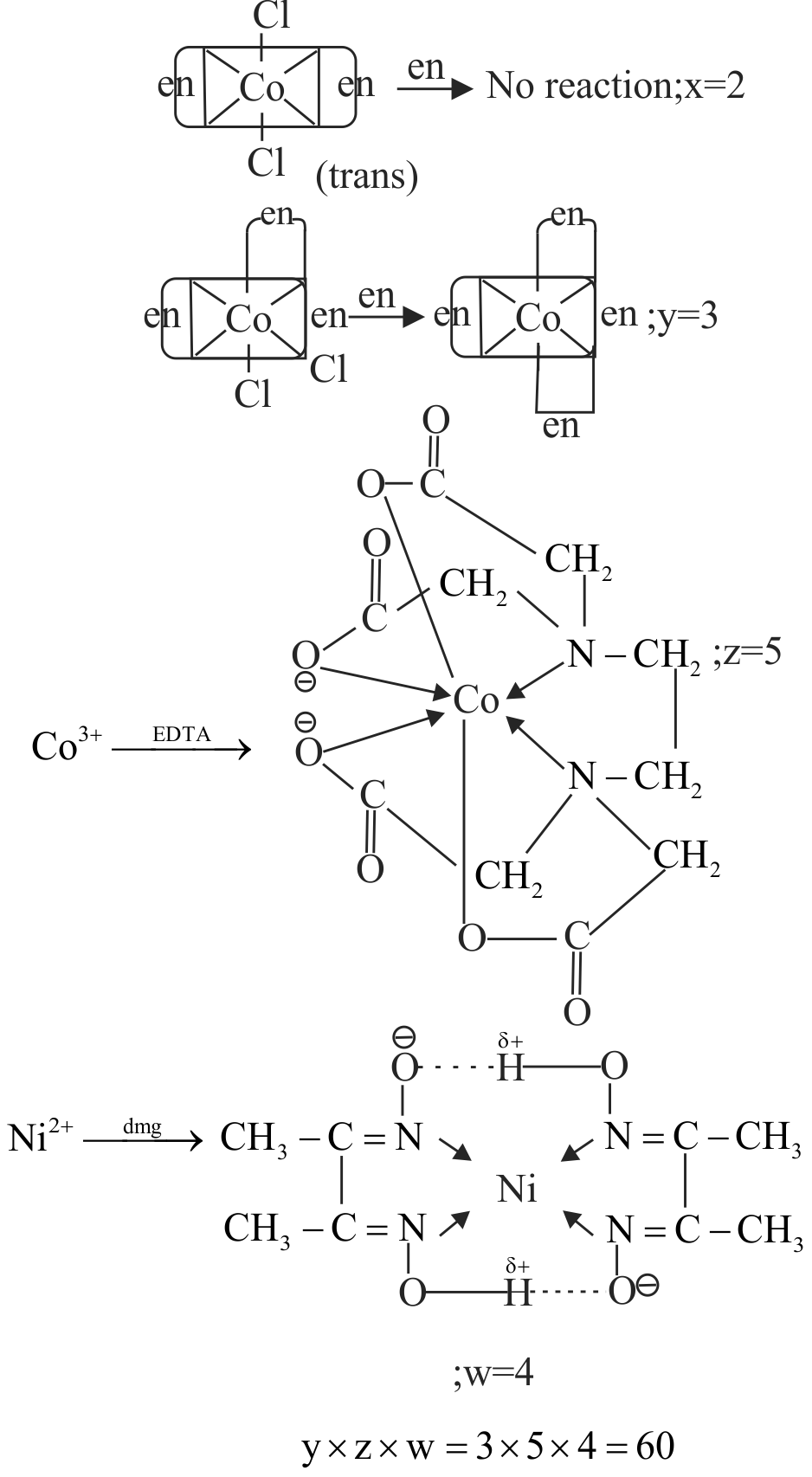
Which is correct for the following reactions?
i. trans \(\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+} \xrightarrow{\text { en }} \mathrm{x}\) (where \(\mathrm{x}\) is the no. of rings in final product)
ii. cis \(\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+} \xrightarrow{\text { en }} \mathrm{y}\) (where \(\mathrm{y}\) is the no. of rings in resulting complex)
iii. \(\mathrm{Co}^{3+} \xrightarrow{\mathrm{EDTA}^{4-}} \mathrm{Z}\) (where \(\mathrm{z}\) is the no. of rings in resulting complex)
iv. \(\mathrm{Ni}^{2+} \xrightarrow{\mathrm{dmg}} \mathrm{W}\) (where \(\mathrm{w}\) is the no. of rings in resulting complex)
322091 In the complexes \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+},\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) \(\left[\mathrm{Fe}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{3}\right]^{3-}\) and \(\left[\mathrm{FeCl}_{6}\right]^{3-}\), more stability is shown by
322094
Which is correct for the following reactions?
i. trans \(\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+} \xrightarrow{\text { en }} \mathrm{x}\) (where \(\mathrm{x}\) is the no. of rings in final product)
ii. cis \(\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+} \xrightarrow{\text { en }} \mathrm{y}\) (where \(\mathrm{y}\) is the no. of rings in resulting complex)
iii. \(\mathrm{Co}^{3+} \xrightarrow{\mathrm{EDTA}^{4-}} \mathrm{Z}\) (where \(\mathrm{z}\) is the no. of rings in resulting complex)
iv. \(\mathrm{Ni}^{2+} \xrightarrow{\mathrm{dmg}} \mathrm{W}\) (where \(\mathrm{w}\) is the no. of rings in resulting complex)
322091 In the complexes \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+},\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) \(\left[\mathrm{Fe}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{3}\right]^{3-}\) and \(\left[\mathrm{FeCl}_{6}\right]^{3-}\), more stability is shown by
322094
Which is correct for the following reactions?
i. trans \(\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+} \xrightarrow{\text { en }} \mathrm{x}\) (where \(\mathrm{x}\) is the no. of rings in final product)
ii. cis \(\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+} \xrightarrow{\text { en }} \mathrm{y}\) (where \(\mathrm{y}\) is the no. of rings in resulting complex)
iii. \(\mathrm{Co}^{3+} \xrightarrow{\mathrm{EDTA}^{4-}} \mathrm{Z}\) (where \(\mathrm{z}\) is the no. of rings in resulting complex)
iv. \(\mathrm{Ni}^{2+} \xrightarrow{\mathrm{dmg}} \mathrm{W}\) (where \(\mathrm{w}\) is the no. of rings in resulting complex)
322091 In the complexes \(\left[\mathrm{Fe}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{3+},\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}\) \(\left[\mathrm{Fe}\left(\mathrm{C}_{2} \mathrm{O}_{4}\right)_{3}\right]^{3-}\) and \(\left[\mathrm{FeCl}_{6}\right]^{3-}\), more stability is shown by
322094
Which is correct for the following reactions?
i. trans \(\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+} \xrightarrow{\text { en }} \mathrm{x}\) (where \(\mathrm{x}\) is the no. of rings in final product)
ii. cis \(\left[\mathrm{Co}(\mathrm{en})_{2} \mathrm{Cl}_{2}\right]^{+} \xrightarrow{\text { en }} \mathrm{y}\) (where \(\mathrm{y}\) is the no. of rings in resulting complex)
iii. \(\mathrm{Co}^{3+} \xrightarrow{\mathrm{EDTA}^{4-}} \mathrm{Z}\) (where \(\mathrm{z}\) is the no. of rings in resulting complex)
iv. \(\mathrm{Ni}^{2+} \xrightarrow{\mathrm{dmg}} \mathrm{W}\) (where \(\mathrm{w}\) is the no. of rings in resulting complex)