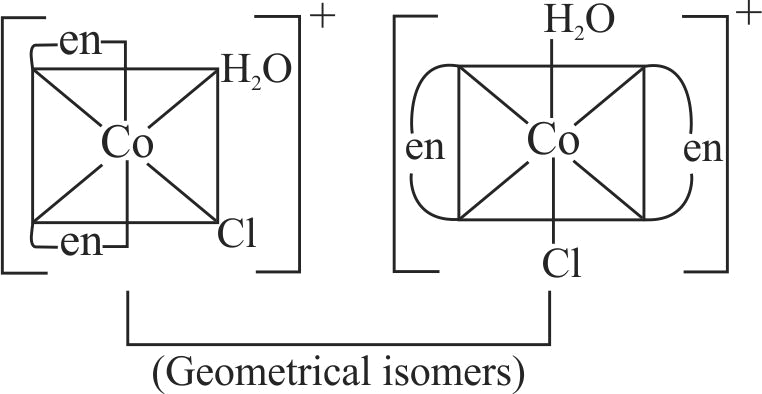
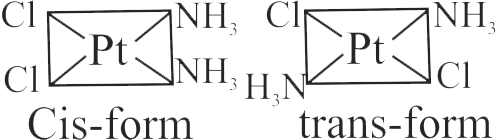321950 \(\left[\mathrm{CoCl}_{2}\left(\mathrm{NH}_{3}\right)_{4}\right]^{+}+\mathrm{Cl}^{-} \rightarrow\left[\mathrm{CoCl}_{3}\left(\mathrm{NH}_{3}\right)_{3}\right]+\mathrm{NH}_{3}\) If in this reaction two isomers of the product are obtained, which is true for the initial reactant complex?
321950 \(\left[\mathrm{CoCl}_{2}\left(\mathrm{NH}_{3}\right)_{4}\right]^{+}+\mathrm{Cl}^{-} \rightarrow\left[\mathrm{CoCl}_{3}\left(\mathrm{NH}_{3}\right)_{3}\right]+\mathrm{NH}_{3}\) If in this reaction two isomers of the product are obtained, which is true for the initial reactant complex?
321950 \(\left[\mathrm{CoCl}_{2}\left(\mathrm{NH}_{3}\right)_{4}\right]^{+}+\mathrm{Cl}^{-} \rightarrow\left[\mathrm{CoCl}_{3}\left(\mathrm{NH}_{3}\right)_{3}\right]+\mathrm{NH}_{3}\) If in this reaction two isomers of the product are obtained, which is true for the initial reactant complex?
321950 \(\left[\mathrm{CoCl}_{2}\left(\mathrm{NH}_{3}\right)_{4}\right]^{+}+\mathrm{Cl}^{-} \rightarrow\left[\mathrm{CoCl}_{3}\left(\mathrm{NH}_{3}\right)_{3}\right]+\mathrm{NH}_{3}\) If in this reaction two isomers of the product are obtained, which is true for the initial reactant complex?