321945
Assertion :
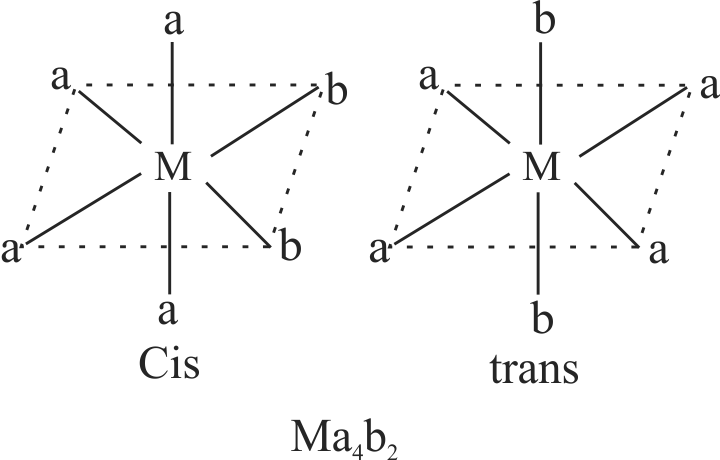
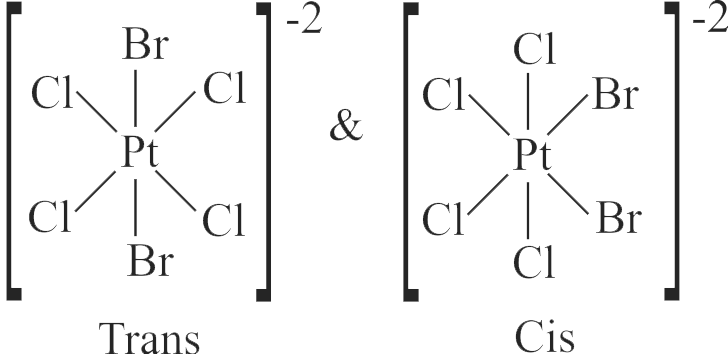
The geometrical isomers of the complex \(\left[\mathrm{M}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]\) are optically inactive.
Reason :
Both geometrical isomers of the complex \(\left[\mathrm{M}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]\) possess axis of symmetry.
321947 The metal atom present in the complex MABXL (where \({\mathrm{\mathrm{A}, \mathrm{B}, \mathrm{X}}}\) and L are unidentate ligands and M is metal) involves \({\mathrm{\mathrm{sp}^{3}}}\) hybridisation. The number of geometrical isomers exhibited by the complex is
321945
Assertion :
The geometrical isomers of the complex \(\left[\mathrm{M}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]\) are optically inactive.
Reason :
Both geometrical isomers of the complex \(\left[\mathrm{M}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]\) possess axis of symmetry.
321947 The metal atom present in the complex MABXL (where \({\mathrm{\mathrm{A}, \mathrm{B}, \mathrm{X}}}\) and L are unidentate ligands and M is metal) involves \({\mathrm{\mathrm{sp}^{3}}}\) hybridisation. The number of geometrical isomers exhibited by the complex is
321945
Assertion :
The geometrical isomers of the complex \(\left[\mathrm{M}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]\) are optically inactive.
Reason :
Both geometrical isomers of the complex \(\left[\mathrm{M}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]\) possess axis of symmetry.
321947 The metal atom present in the complex MABXL (where \({\mathrm{\mathrm{A}, \mathrm{B}, \mathrm{X}}}\) and L are unidentate ligands and M is metal) involves \({\mathrm{\mathrm{sp}^{3}}}\) hybridisation. The number of geometrical isomers exhibited by the complex is
321945
Assertion :
The geometrical isomers of the complex \(\left[\mathrm{M}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]\) are optically inactive.
Reason :
Both geometrical isomers of the complex \(\left[\mathrm{M}\left(\mathrm{NH}_{3}\right)_{4} \mathrm{Cl}_{2}\right]\) possess axis of symmetry.
321947 The metal atom present in the complex MABXL (where \({\mathrm{\mathrm{A}, \mathrm{B}, \mathrm{X}}}\) and L are unidentate ligands and M is metal) involves \({\mathrm{\mathrm{sp}^{3}}}\) hybridisation. The number of geometrical isomers exhibited by the complex is