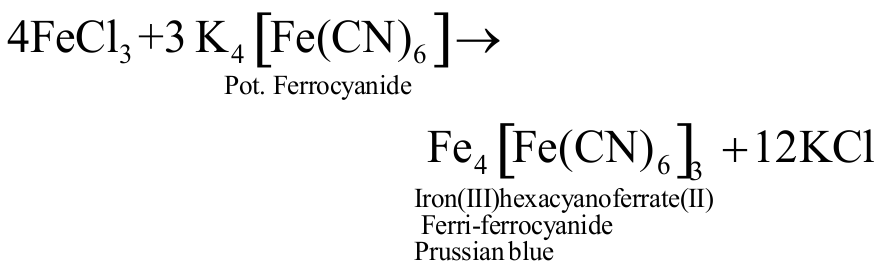321901
Read the Statement - A and Statement - B carefully to mark the correct options given below
Statement A :
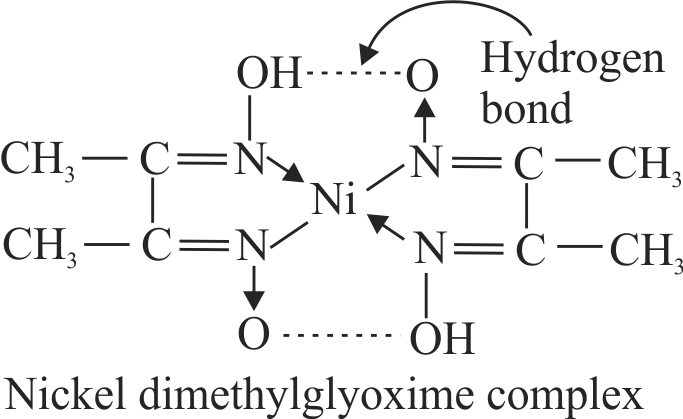
Dimethyl glyoxime forms a sixmembered covalent chelate when treated with \({\mathrm{\mathrm{NiCl}_{2}}}\) solution in presence of \({\mathrm{\mathrm{NH}_{4} \mathrm{OH}}}\).
Statement B :
Prussian blue precipitate contains iron both in \({\mathrm{(+2)}}\) and \({\mathrm{(+3)}}\) oxidation states.
321901
Read the Statement - A and Statement - B carefully to mark the correct options given below
Statement A :
Dimethyl glyoxime forms a sixmembered covalent chelate when treated with \({\mathrm{\mathrm{NiCl}_{2}}}\) solution in presence of \({\mathrm{\mathrm{NH}_{4} \mathrm{OH}}}\).
Statement B :
Prussian blue precipitate contains iron both in \({\mathrm{(+2)}}\) and \({\mathrm{(+3)}}\) oxidation states.
321901
Read the Statement - A and Statement - B carefully to mark the correct options given below
Statement A :
Dimethyl glyoxime forms a sixmembered covalent chelate when treated with \({\mathrm{\mathrm{NiCl}_{2}}}\) solution in presence of \({\mathrm{\mathrm{NH}_{4} \mathrm{OH}}}\).
Statement B :
Prussian blue precipitate contains iron both in \({\mathrm{(+2)}}\) and \({\mathrm{(+3)}}\) oxidation states.
321901
Read the Statement - A and Statement - B carefully to mark the correct options given below
Statement A :
Dimethyl glyoxime forms a sixmembered covalent chelate when treated with \({\mathrm{\mathrm{NiCl}_{2}}}\) solution in presence of \({\mathrm{\mathrm{NH}_{4} \mathrm{OH}}}\).
Statement B :
Prussian blue precipitate contains iron both in \({\mathrm{(+2)}}\) and \({\mathrm{(+3)}}\) oxidation states.