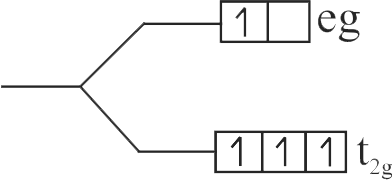
321859 The value of \(\Delta \) for \({[{\rm{Rh(C}}{{\rm{l}}_{\rm{6}}}{\rm{)}}]^{3 - }}\) is \(243\,{\rm{kJ}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}.\) Then the wavelength of the light which will promote an electron from the \({{\rm{t}}_{{\rm{2g}}}}\) set to \({{\rm{e}}_{\rm{g}}}\) set is ____.
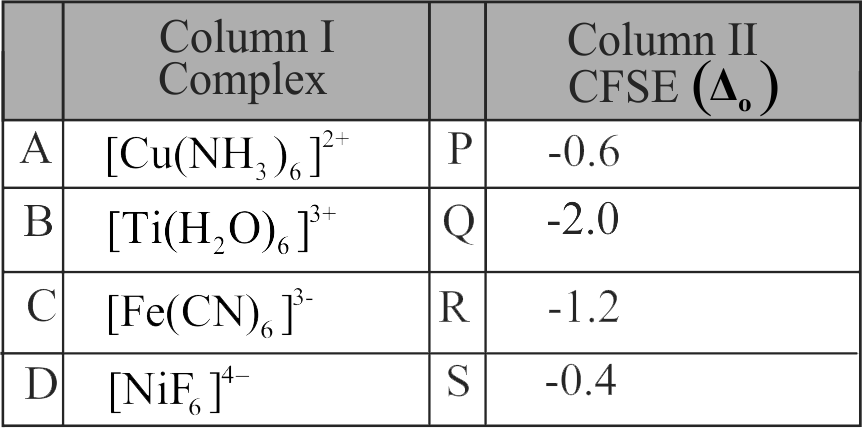
321861 Arrange following complex ions in increasing order of crystal field splitting energy \(\left(\Delta_{0}\right)\) : \(\left[\mathrm{Cr}(\mathrm{Cl})_{6}\right]^{3-},\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]^{3-},\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\).
321859 The value of \(\Delta \) for \({[{\rm{Rh(C}}{{\rm{l}}_{\rm{6}}}{\rm{)}}]^{3 - }}\) is \(243\,{\rm{kJ}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}.\) Then the wavelength of the light which will promote an electron from the \({{\rm{t}}_{{\rm{2g}}}}\) set to \({{\rm{e}}_{\rm{g}}}\) set is ____.
321861 Arrange following complex ions in increasing order of crystal field splitting energy \(\left(\Delta_{0}\right)\) : \(\left[\mathrm{Cr}(\mathrm{Cl})_{6}\right]^{3-},\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]^{3-},\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\).
321859 The value of \(\Delta \) for \({[{\rm{Rh(C}}{{\rm{l}}_{\rm{6}}}{\rm{)}}]^{3 - }}\) is \(243\,{\rm{kJ}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}.\) Then the wavelength of the light which will promote an electron from the \({{\rm{t}}_{{\rm{2g}}}}\) set to \({{\rm{e}}_{\rm{g}}}\) set is ____.
321861 Arrange following complex ions in increasing order of crystal field splitting energy \(\left(\Delta_{0}\right)\) : \(\left[\mathrm{Cr}(\mathrm{Cl})_{6}\right]^{3-},\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]^{3-},\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\).
321859 The value of \(\Delta \) for \({[{\rm{Rh(C}}{{\rm{l}}_{\rm{6}}}{\rm{)}}]^{3 - }}\) is \(243\,{\rm{kJ}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}.\) Then the wavelength of the light which will promote an electron from the \({{\rm{t}}_{{\rm{2g}}}}\) set to \({{\rm{e}}_{\rm{g}}}\) set is ____.
321861 Arrange following complex ions in increasing order of crystal field splitting energy \(\left(\Delta_{0}\right)\) : \(\left[\mathrm{Cr}(\mathrm{Cl})_{6}\right]^{3-},\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]^{3-},\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\).
321859 The value of \(\Delta \) for \({[{\rm{Rh(C}}{{\rm{l}}_{\rm{6}}}{\rm{)}}]^{3 - }}\) is \(243\,{\rm{kJ}}\,{\rm{mo}}{{\rm{l}}^{ - 1}}.\) Then the wavelength of the light which will promote an electron from the \({{\rm{t}}_{{\rm{2g}}}}\) set to \({{\rm{e}}_{\rm{g}}}\) set is ____.
321861 Arrange following complex ions in increasing order of crystal field splitting energy \(\left(\Delta_{0}\right)\) : \(\left[\mathrm{Cr}(\mathrm{Cl})_{6}\right]^{3-},\left[\mathrm{Cr}(\mathrm{CN})_{6}\right]^{3-},\left[\mathrm{Cr}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\).