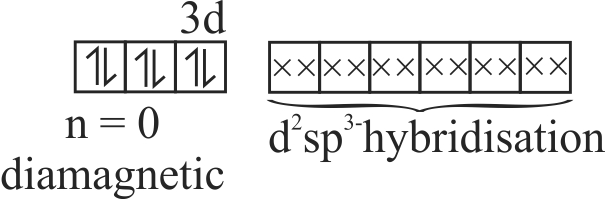321846 In the crystal field of the complex \(\left[\mathrm{Fe}(\mathrm{Cl})(\mathrm{CN})_{4}\left(\mathrm{O}_{2}\right)\right]^{4-}\) the electronic configuration of metal is found to be \({\text{t}}_{{\text{2g}}}^{\text{6}}{\text{,e}}_{\text{g}}^{\text{0}}\) Then which of the following is true about this complexion:
321848
The increasing order of the value is \(\operatorname{CFSE}\left(\Delta_{o}\right)\) for the following species is
I. \(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
II. \(\left[\mathrm{Rh}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
III. \(\left[\operatorname{Ir}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
321849
The number of complexes from the following with no electrons in the \({\mathrm{t_{2}}}\) orbital is ____.
\({\mathrm{\mathrm{TiCl}_{4},\left[\mathrm{MnO}_{4}\right]^{-},\left[\mathrm{FeO}_{4}\right]^{2-},\left[\mathrm{FeCl}_{4}\right]^{-},\left[\mathrm{CoCl}_{4}\right]^{2-}}}\)
321846 In the crystal field of the complex \(\left[\mathrm{Fe}(\mathrm{Cl})(\mathrm{CN})_{4}\left(\mathrm{O}_{2}\right)\right]^{4-}\) the electronic configuration of metal is found to be \({\text{t}}_{{\text{2g}}}^{\text{6}}{\text{,e}}_{\text{g}}^{\text{0}}\) Then which of the following is true about this complexion:
321848
The increasing order of the value is \(\operatorname{CFSE}\left(\Delta_{o}\right)\) for the following species is
I. \(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
II. \(\left[\mathrm{Rh}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
III. \(\left[\operatorname{Ir}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
321849
The number of complexes from the following with no electrons in the \({\mathrm{t_{2}}}\) orbital is ____.
\({\mathrm{\mathrm{TiCl}_{4},\left[\mathrm{MnO}_{4}\right]^{-},\left[\mathrm{FeO}_{4}\right]^{2-},\left[\mathrm{FeCl}_{4}\right]^{-},\left[\mathrm{CoCl}_{4}\right]^{2-}}}\)
321846 In the crystal field of the complex \(\left[\mathrm{Fe}(\mathrm{Cl})(\mathrm{CN})_{4}\left(\mathrm{O}_{2}\right)\right]^{4-}\) the electronic configuration of metal is found to be \({\text{t}}_{{\text{2g}}}^{\text{6}}{\text{,e}}_{\text{g}}^{\text{0}}\) Then which of the following is true about this complexion:
321848
The increasing order of the value is \(\operatorname{CFSE}\left(\Delta_{o}\right)\) for the following species is
I. \(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
II. \(\left[\mathrm{Rh}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
III. \(\left[\operatorname{Ir}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
321849
The number of complexes from the following with no electrons in the \({\mathrm{t_{2}}}\) orbital is ____.
\({\mathrm{\mathrm{TiCl}_{4},\left[\mathrm{MnO}_{4}\right]^{-},\left[\mathrm{FeO}_{4}\right]^{2-},\left[\mathrm{FeCl}_{4}\right]^{-},\left[\mathrm{CoCl}_{4}\right]^{2-}}}\)
321846 In the crystal field of the complex \(\left[\mathrm{Fe}(\mathrm{Cl})(\mathrm{CN})_{4}\left(\mathrm{O}_{2}\right)\right]^{4-}\) the electronic configuration of metal is found to be \({\text{t}}_{{\text{2g}}}^{\text{6}}{\text{,e}}_{\text{g}}^{\text{0}}\) Then which of the following is true about this complexion:
321848
The increasing order of the value is \(\operatorname{CFSE}\left(\Delta_{o}\right)\) for the following species is
I. \(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
II. \(\left[\mathrm{Rh}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
III. \(\left[\operatorname{Ir}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
321849
The number of complexes from the following with no electrons in the \({\mathrm{t_{2}}}\) orbital is ____.
\({\mathrm{\mathrm{TiCl}_{4},\left[\mathrm{MnO}_{4}\right]^{-},\left[\mathrm{FeO}_{4}\right]^{2-},\left[\mathrm{FeCl}_{4}\right]^{-},\left[\mathrm{CoCl}_{4}\right]^{2-}}}\)
321846 In the crystal field of the complex \(\left[\mathrm{Fe}(\mathrm{Cl})(\mathrm{CN})_{4}\left(\mathrm{O}_{2}\right)\right]^{4-}\) the electronic configuration of metal is found to be \({\text{t}}_{{\text{2g}}}^{\text{6}}{\text{,e}}_{\text{g}}^{\text{0}}\) Then which of the following is true about this complexion:
321848
The increasing order of the value is \(\operatorname{CFSE}\left(\Delta_{o}\right)\) for the following species is
I. \(\left[\mathrm{Co}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
II. \(\left[\mathrm{Rh}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
III. \(\left[\operatorname{Ir}\left(\mathrm{NH}_{3}\right)_{6}\right]^{3+}\)
321849
The number of complexes from the following with no electrons in the \({\mathrm{t_{2}}}\) orbital is ____.
\({\mathrm{\mathrm{TiCl}_{4},\left[\mathrm{MnO}_{4}\right]^{-},\left[\mathrm{FeO}_{4}\right]^{2-},\left[\mathrm{FeCl}_{4}\right]^{-},\left[\mathrm{CoCl}_{4}\right]^{2-}}}\)