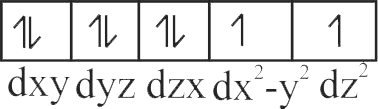321835
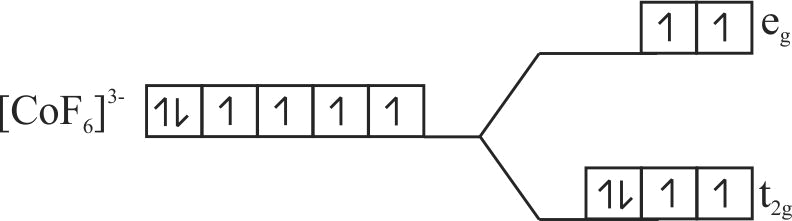
Using crystal field theory electronic configuration of the central metal atom/ion and the magnetic moment value in the following:
\(\left[\mathrm{CoF}_{6}\right]^{3-},\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+},\left[\mathrm{Co}(\mathrm{CN})_{6}\right]^{3-}\)
321835
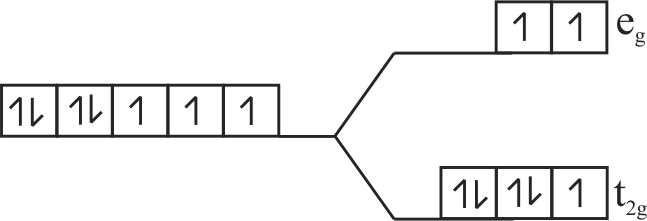
Using crystal field theory electronic configuration of the central metal atom/ion and the magnetic moment value in the following:
\(\left[\mathrm{CoF}_{6}\right]^{3-},\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+},\left[\mathrm{Co}(\mathrm{CN})_{6}\right]^{3-}\)
321835
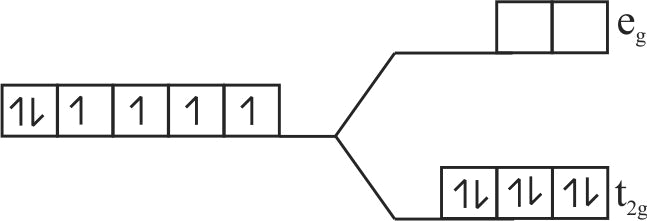
Using crystal field theory electronic configuration of the central metal atom/ion and the magnetic moment value in the following:
\(\left[\mathrm{CoF}_{6}\right]^{3-},\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+},\left[\mathrm{Co}(\mathrm{CN})_{6}\right]^{3-}\)
321835
Using crystal field theory electronic configuration of the central metal atom/ion and the magnetic moment value in the following:
\(\left[\mathrm{CoF}_{6}\right]^{3-},\left[\mathrm{Co}\left(\mathrm{H}_{2} \mathrm{O}\right)_{6}\right]^{2+},\left[\mathrm{Co}(\mathrm{CN})_{6}\right]^{3-}\)